Heat And Temperature Answer Key . It is not always easy to distinguish these terms. Answer key heat practice problems q = m x ∆t x c 1. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. However, in physics, although they are related, these terms are not the same. 5.0 g of copper was heated from 20°c to 80°c. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. In this chapter, we explore heat and temperature. How much energy was used to heat cu?. This lowers the temperature of water by \ (\displaystyle δt_2:. People think that heat and temperature are the same. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): Show all work and proper. Heat is the flow of energy from one object to.
from amiahgokeestes.blogspot.com
Heat is the flow of energy from one object to. It is not always easy to distinguish these terms. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. 5.0 g of copper was heated from 20°c to 80°c. However, in physics, although they are related, these terms are not the same. Show all work and proper. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. In this chapter, we explore heat and temperature. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): This lowers the temperature of water by \ (\displaystyle δt_2:.
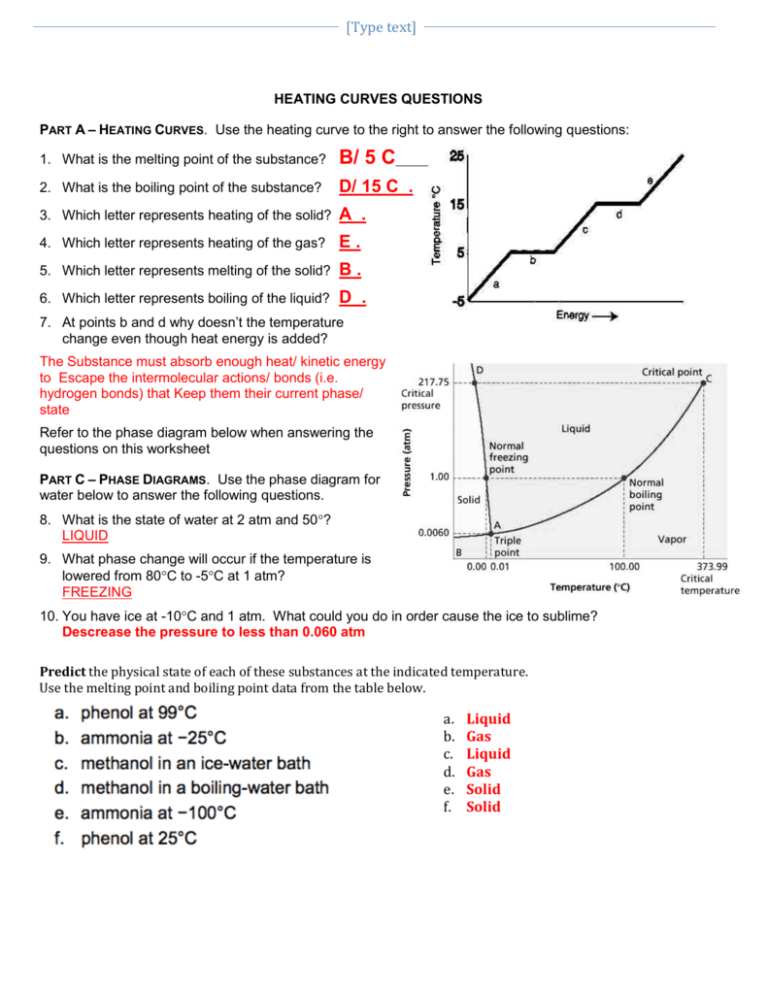
Use the Heating Curve Below to Answer the Following Questions
Heat And Temperature Answer Key It is not always easy to distinguish these terms. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. 5.0 g of copper was heated from 20°c to 80°c. It is not always easy to distinguish these terms. Answer key heat practice problems q = m x ∆t x c 1. In this chapter, we explore heat and temperature. Show all work and proper. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. This lowers the temperature of water by \ (\displaystyle δt_2:. However, in physics, although they are related, these terms are not the same. Heat is the flow of energy from one object to. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): People think that heat and temperature are the same. How much energy was used to heat cu?.
From askworksheet.com
Thermal Energy Temperature And Heat Worksheet Lesson 1 Answer Key Heat And Temperature Answer Key This lowers the temperature of water by \ (\displaystyle δt_2:. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. In this chapter, we explore heat and temperature. How much energy was used to. Heat And Temperature Answer Key.
From www.vrogue.co
Best Temperature Conversion Worksheet Answers Tempera vrogue.co Heat And Temperature Answer Key Answer key heat practice problems q = m x ∆t x c 1. However, in physics, although they are related, these terms are not the same. In this chapter, we explore heat and temperature. Heat is the flow of energy from one object to. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): People. Heat And Temperature Answer Key.
From studyfinder.org
Unraveling the Answers A Comprehensive Look at Chapter 21 Heat And Temperature Answer Key However, in physics, although they are related, these terms are not the same. How much energy was used to heat cu?. It is not always easy to distinguish these terms. Answer key heat practice problems q = m x ∆t x c 1. People think that heat and temperature are the same. Heat is the flow of energy from one. Heat And Temperature Answer Key.
From www.worksheeto.com
12 Best Images of Heat And Temperature Worksheets Temperature and Heat And Temperature Answer Key Heat is the flow of energy from one object to. It is not always easy to distinguish these terms. 5.0 g of copper was heated from 20°c to 80°c. How much energy was used to heat cu?. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): However, in physics, although they are related, these. Heat And Temperature Answer Key.
From www.studocu.com
350156920 Methods of Heat Transfer Answers Worksheet Methods of Heat Heat And Temperature Answer Key It is not always easy to distinguish these terms. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. In this chapter, we explore heat and temperature. However, in physics, although they are related, these terms are not the same. First bring the ice up to 0°c and melt it with heat \ (\displaystyle. Heat And Temperature Answer Key.
From worksheetpic101.s3.amazonaws.com
temperature thermal energy and heat worksheet Heat And Temperature Answer Key Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. Answer key heat practice problems q = m x ∆t x c 1. However, in physics, although they are related, these terms are not the same. 5.0 g of copper was heated from 20°c to 80°c. First bring the. Heat And Temperature Answer Key.
From printablecreative.com
Temperature and Heat Crossword Puzzle Heat And Temperature Answer Key People think that heat and temperature are the same. In this chapter, we explore heat and temperature. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. 5.0 g of copper was heated from 20°c to 80°c. This lowers the temperature of water by \ (\displaystyle δt_2:. Heat is the flow of energy from. Heat And Temperature Answer Key.
From studylib.net
What Is Heat? PostQuiz Answer Key Heat And Temperature Answer Key However, in physics, although they are related, these terms are not the same. This lowers the temperature of water by \ (\displaystyle δt_2:. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Heat is the flow of energy from one object to. First bring the ice up to 0°c and melt it with. Heat And Temperature Answer Key.
From learningfullmaurer.z1.web.core.windows.net
Heat Vs Temperature Worksheet Answer Key Heat And Temperature Answer Key Answer key heat practice problems q = m x ∆t x c 1. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. People think that heat and temperature are the same. This lowers the temperature of water by \ (\displaystyle δt_2:. How much energy was used to heat cu?. Since the internal energy. Heat And Temperature Answer Key.
From kimora-kbowen.blogspot.com
Temperature and Specific Heat Lab 4 Answers Heat And Temperature Answer Key 5.0 g of copper was heated from 20°c to 80°c. Show all work and proper. Heat is the flow of energy from one object to. People think that heat and temperature are the same. How much energy was used to heat cu?. However, in physics, although they are related, these terms are not the same. In this chapter, we explore. Heat And Temperature Answer Key.
From studylib.net
Activity 1.3.3 Thermodynamics Answer Key Heat And Temperature Answer Key Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. This lowers the temperature of water by \ (\displaystyle δt_2:. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): It is not always easy to distinguish these terms. Answer key heat practice problems. Heat And Temperature Answer Key.
From learninglistpropst.z21.web.core.windows.net
Heat Vs Temperature Worksheet Answer Key Heat And Temperature Answer Key People think that heat and temperature are the same. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): However, in physics, although they are related, these terms are not the same. Answer key heat practice problems q = m x ∆t x c 1. It is not always easy to distinguish these terms. In. Heat And Temperature Answer Key.
From www.proworksheet.my.id
Heat And Temperature Worksheet Pro Worksheet Heat And Temperature Answer Key Heat is the flow of energy from one object to. 5.0 g of copper was heated from 20°c to 80°c. Answer key heat practice problems q = m x ∆t x c 1. This lowers the temperature of water by \ (\displaystyle δt_2:. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Since. Heat And Temperature Answer Key.
From kidsworksheetfun.com
Specific Heat Worksheet 2 Answer Key Kidsworksheetfun Heat And Temperature Answer Key Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. How much energy was used to heat cu?. Show all work and proper. It is not always easy to distinguish these terms. In. Heat And Temperature Answer Key.
From www.onlineworksheet.my.id
Specific Heat Worksheet Answer Key Heat And Temperature Answer Key Show all work and proper. People think that heat and temperature are the same. How much energy was used to heat cu?. However, in physics, although they are related, these terms are not the same. Answer key heat practice problems q = m x ∆t x c 1. In this chapter, we explore heat and temperature. Since the internal energy. Heat And Temperature Answer Key.
From quizizz.com
50+ heat transfer and thermal equilibrium worksheets for 10th Grade on Heat And Temperature Answer Key Show all work and proper. How much energy was used to heat cu?. It is not always easy to distinguish these terms. 5.0 g of copper was heated from 20°c to 80°c. This lowers the temperature of water by \ (\displaystyle δt_2:. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal. Heat And Temperature Answer Key.
From worksheets.decoomo.com
10++ Specific Heat Worksheet Answer Key Worksheets Decoomo Heat And Temperature Answer Key It is not always easy to distinguish these terms. In this chapter, we explore heat and temperature. However, in physics, although they are related, these terms are not the same. People think that heat and temperature are the same. Heat is the flow of energy from one object to. How much energy was used to heat cu?. = mc∆t, where. Heat And Temperature Answer Key.
From davida.davivienda.com
Heat Vs Temperature Worksheet Answer Key Printable Word Searches Heat And Temperature Answer Key = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and proper. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): People think that heat and temperature are the same. However, in physics, although they are related, these terms are not the same. In this. Heat And Temperature Answer Key.
From martindxmguide.blogspot.com
34 Specific Heat Worksheet Answer Key support worksheet Heat And Temperature Answer Key This lowers the temperature of water by \ (\displaystyle δt_2:. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): 5.0 g of copper was heated from 20°c to 80°c. It is not always easy to distinguish these terms. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp.. Heat And Temperature Answer Key.
From studylib.net
Back Short Answer Questions on Heat, Temperature and Calorimetry Heat And Temperature Answer Key However, in physics, although they are related, these terms are not the same. Heat is the flow of energy from one object to. Show all work and proper. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): Answer key heat practice problems q = m x ∆t x c 1. How much energy was. Heat And Temperature Answer Key.
From classfullmathew.z13.web.core.windows.net
Temperature Thermal Energy And Heat Worksheets Answer Key Heat And Temperature Answer Key Show all work and proper. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. This lowers the temperature of water by \ (\displaystyle δt_2:. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. Answer key heat practice problems q = m. Heat And Temperature Answer Key.
From www.e-streetlight.com
Heat And Temperature Worksheet E Street Light Heat And Temperature Answer Key = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. This lowers the temperature of water by \ (\displaystyle δt_2:. 5.0 g of copper was heated from 20°c to 80°c. How much energy was used to heat cu?. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): However,. Heat And Temperature Answer Key.
From www.studocu.com
Activity 1 Temperature and Heat ACTIVITY 1 SESE 119 (Thermodynamics Heat And Temperature Answer Key 5.0 g of copper was heated from 20°c to 80°c. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. It is not always easy to distinguish these terms. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): Show all work and proper.. Heat And Temperature Answer Key.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Heat And Temperature Answer Key People think that heat and temperature are the same. This lowers the temperature of water by \ (\displaystyle δt_2:. It is not always easy to distinguish these terms. Heat is the flow of energy from one object to. Show all work and proper. In this chapter, we explore heat and temperature. = mc∆t, where q = heat energy, m =. Heat And Temperature Answer Key.
From www.pdffiller.com
Section 16 1 Thermal Energy And Matter Fill Online, Printable Heat And Temperature Answer Key Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. This lowers the temperature of water by \ (\displaystyle δt_2:. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): In this chapter, we explore heat and temperature. = mc∆t, where q = heat. Heat And Temperature Answer Key.
From amiahgokeestes.blogspot.com
Use the Heating Curve Below to Answer the Following Questions Heat And Temperature Answer Key First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): This lowers the temperature of water by \ (\displaystyle δt_2:. Answer key heat practice problems q = m x ∆t x c 1. It is not always easy to distinguish these terms. How much energy was used to heat cu?. However, in physics, although they. Heat And Temperature Answer Key.
From davida.davivienda.com
Heat Vs Temperature Worksheet Answer Key Printable Word Searches Heat And Temperature Answer Key It is not always easy to distinguish these terms. Heat is the flow of energy from one object to. Answer key heat practice problems q = m x ∆t x c 1. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. First bring the ice up to 0°c. Heat And Temperature Answer Key.
From www.englishworksheet.my.id
Heat And Temperature Worksheet English Worksheet Heat And Temperature Answer Key Heat is the flow of energy from one object to. Show all work and proper. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): How much energy was used to heat cu?. It is not always easy to distinguish these terms. Since the internal energy is directly proportional to the temperature, the hot cup. Heat And Temperature Answer Key.
From printablezonebunias.z21.web.core.windows.net
Heat And Temperature Worksheet Answer Key Heat And Temperature Answer Key It is not always easy to distinguish these terms. Heat is the flow of energy from one object to. This lowers the temperature of water by \ (\displaystyle δt_2:. Answer key heat practice problems q = m x ∆t x c 1. 5.0 g of copper was heated from 20°c to 80°c. Show all work and proper. Since the internal. Heat And Temperature Answer Key.
From misstagore.com
Heat key words mind map Mrs Tagore's Science Page Heat And Temperature Answer Key First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. People think that heat and temperature are the same. Answer key heat practice problems q = m x ∆t x c 1. 5.0 g of copper was heated from. Heat And Temperature Answer Key.
From www.worksheeto.com
12 Best Images of Heat And Temperature Worksheets Temperature and Heat And Temperature Answer Key This lowers the temperature of water by \ (\displaystyle δt_2:. It is not always easy to distinguish these terms. However, in physics, although they are related, these terms are not the same. Show all work and proper. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): Since the internal energy is directly proportional to. Heat And Temperature Answer Key.
From learninglistpropst.z21.web.core.windows.net
Heat Vs Temperature Worksheet Answer Key Heat And Temperature Answer Key This lowers the temperature of water by \ (\displaystyle δt_2:. People think that heat and temperature are the same. However, in physics, although they are related, these terms are not the same. 5.0 g of copper was heated from 20°c to 80°c. It is not always easy to distinguish these terms. Heat is the flow of energy from one object. Heat And Temperature Answer Key.
From www.e-streetlight.com
Heat And Temperature Worksheet E Street Light Heat And Temperature Answer Key = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and proper. This lowers the temperature of water by \ (\displaystyle δt_2:. People think that heat and temperature are the same. How much energy was used to heat cu?. Answer key heat practice problems q = m x ∆t x c. Heat And Temperature Answer Key.
From studylib.net
Calculating Heat ANSWER KEY Heat And Temperature Answer Key First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): How much energy was used to heat cu?. Answer key heat practice problems q = m x ∆t x c 1. In this chapter, we explore heat and temperature. However, in physics, although they are related, these terms are not the same. It is not. Heat And Temperature Answer Key.
From www.worksheeto.com
17 Temperature And Heat Worksheet Answers / Heat And Temperature Answer Key Show all work and proper. Heat is the flow of energy from one object to. First bring the ice up to 0°c and melt it with heat \ (\displaystyle q_1\): In this chapter, we explore heat and temperature. Since the internal energy is directly proportional to the temperature, the hot cup of coffee has higher internal energy compared to. However,. Heat And Temperature Answer Key.