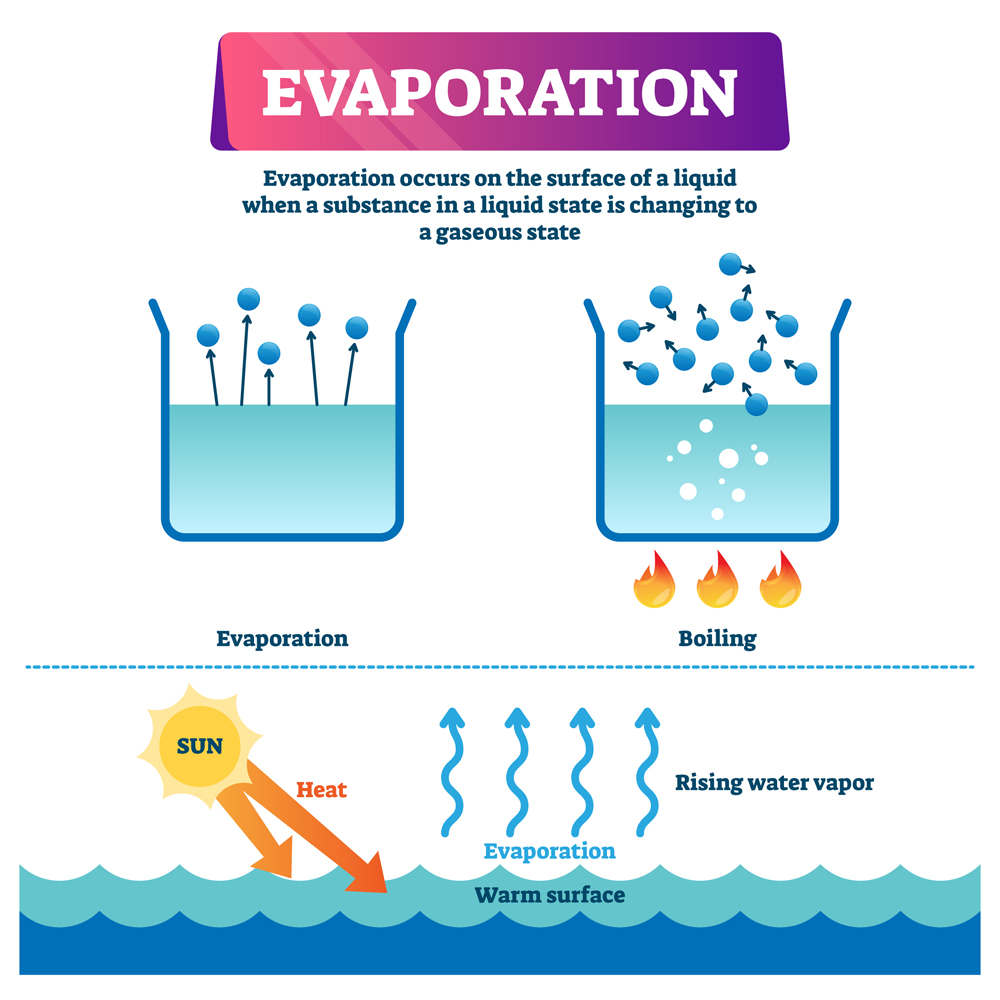
Why Does Water Evaporate Fast . The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation hasn’t been studied in detail at. What is evaporation and why does it occur? Evaporation is the process that changes liquid water to gaseous water (water vapor). Water moves from the earth’s surface to the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. When liquid water meets dry air, it is not in equilibrium; The level of humidity in the air also plays a role in the process of evaporation. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere.
from www.scienceabc.com
The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. What is evaporation and why does it occur? The level of humidity in the air also plays a role in the process of evaporation. Water moves from the earth’s surface to the. Evaporation is the process that changes liquid water to gaseous water (water vapor). Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates.
Why Does Water Evaporate At Room Temperature?
Why Does Water Evaporate Fast Evaporation is the process that changes liquid water to gaseous water (water vapor). Evaporation is the process that changes liquid water to gaseous water (water vapor). Water moves from the earth’s surface to the. Evaporation hasn’t been studied in detail at. What is evaporation and why does it occur? The level of humidity in the air also plays a role in the process of evaporation. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. When liquid water meets dry air, it is not in equilibrium;
From efishkeeping.com
Top 6 Reasons Why Fish Tank Water Evaporates (+ Fixes!) eFishkeeping Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as. Why Does Water Evaporate Fast.
From schematicparttod.z21.web.core.windows.net
Why Does Oil Not Evaporate Why Does Water Evaporate Fast Evaporation is the process that changes liquid water to gaseous water (water vapor). Evaporation hasn’t been studied in detail at. When liquid water meets dry air, it is not in equilibrium; Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. Water molecules evaporate off the surface until the amount of water in. Why Does Water Evaporate Fast.
From fyocmlcvg.blob.core.windows.net
Does Water Evaporate Out Of Pool at Ronnie Hawk blog Why Does Water Evaporate Fast Water moves from the earth’s surface to the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. What is evaporation and why does it occur? Evaporation is the process that changes liquid water to gaseous water (water vapor). The level of humidity in the air also plays a role. Why Does Water Evaporate Fast.
From www.youtube.com
Why does water evaporate at room temp? YouTube Why Does Water Evaporate Fast When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. What is evaporation and why does it occur? Evaporation hasn’t been studied in detail at. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The level of humidity in the. Why Does Water Evaporate Fast.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Why Does Water Evaporate Fast Evaporation hasn’t been studied in detail at. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. Evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. What is evaporation and why does. Why Does Water Evaporate Fast.
From sciencing.com
Fast Ways to Make Water Evaporate Sciencing Why Does Water Evaporate Fast Water moves from the earth’s surface to the. When liquid water meets dry air, it is not in equilibrium; Evaporation is the process that changes liquid water to gaseous water (water vapor). Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water molecules evaporate off the surface. Why Does Water Evaporate Fast.
From schematicparttod.z21.web.core.windows.net
Why Does Oil Not Evaporate Why Does Water Evaporate Fast Evaporation hasn’t been studied in detail at. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water moves from the earth’s surface to the. Evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain. Why Does Water Evaporate Fast.
From www.reddit.com
ELI5 why does water evaporate even when it's not at its boiling point Why Does Water Evaporate Fast The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. When liquid water meets dry air, it is not in equilibrium; Evaporation is the process that changes liquid water to gaseous water (water vapor). Evaporation hasn’t been studied in detail at. What is evaporation and why does it occur? Water moves. Why Does Water Evaporate Fast.
From www.youtube.com
Science Experiment Episode. 5 Water DOES NOT EVAPORATE!!! YouTube Why Does Water Evaporate Fast Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. What is evaporation and why does it occur? When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The pressure pushing down on the water makes it difficult for water to. Why Does Water Evaporate Fast.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Why Does Water Evaporate Fast Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. What is evaporation and why does it occur? The level of humidity in the air also plays a role in the process of evaporation. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker. Why Does Water Evaporate Fast.
From www.youtube.com
Does salt in salt water evaporate? YouTube Why Does Water Evaporate Fast Water moves from the earth’s surface to the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Evaporation hasn’t been studied in detail at. When liquid water meets dry air, it is not in equilibrium; When clothes are hung on a laundry line, even though the ambient temperature is. Why Does Water Evaporate Fast.
From www.archyde.com
Why does gasoline evaporate quickly from cars? Archyde Why Does Water Evaporate Fast Water moves from the earth’s surface to the. Evaporation is the process that changes liquid water to gaseous water (water vapor). When liquid water meets dry air, it is not in equilibrium; When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. When the surface is exposed to. Why Does Water Evaporate Fast.
From www.pinterest.com
Does Cold Water Boil Faster Than Warm Water in 2022 Water boiling Why Does Water Evaporate Fast The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. What is evaporation and why does it occur? Changes in. Why Does Water Evaporate Fast.
From acquadom.com
Why Does My Aquarium Water Evaporate So Fast AcquaDom Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. What is evaporation and why does it occur? Evaporation hasn’t been studied in detail at. The level of humidity in the air also plays a role in the process of evaporation. When liquid water meets dry air, it. Why Does Water Evaporate Fast.
From musicbykatie.com
Does Adding Alcohol To Water Make It Evaporate Faster? The 15 Detailed Why Does Water Evaporate Fast When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. Evaporation is the process that changes liquid water to gaseous water (water vapor). What is evaporation and why does it occur? When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere.. Why Does Water Evaporate Fast.
From www.amazon.ca
Why Does Water Evaporate? All about Heat and Temperature Moore, Rob Why Does Water Evaporate Fast Evaporation hasn’t been studied in detail at. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. What is evaporation and why does it occur? When. Why Does Water Evaporate Fast.
From gionlypja.blob.core.windows.net
Why Does Only Water Evaporate at Deidre Watts blog Why Does Water Evaporate Fast Evaporation is the process that changes liquid water to gaseous water (water vapor). Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When liquid water meets dry air, it is not in equilibrium; When clothes are hung on a laundry line, even though the ambient temperature is. Why Does Water Evaporate Fast.
From foodwine.com
How Fast Does Alcohol Evaporate [At Room Temperature and When Boiling Why Does Water Evaporate Fast When liquid water meets dry air, it is not in equilibrium; Evaporation hasn’t been studied in detail at. Evaporation is the process that changes liquid water to gaseous water (water vapor). The level of humidity in the air also plays a role in the process of evaporation. What is evaporation and why does it occur? Changes in pressure, more so. Why Does Water Evaporate Fast.
From studylibbasinets.z21.web.core.windows.net
Why Does The Water Evaporate Why Does Water Evaporate Fast What is evaporation and why does it occur? When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. When liquid water meets dry air, it is not in equilibrium; Water moves from the earth’s surface to the. The level of humidity in the air also plays a role. Why Does Water Evaporate Fast.
From fyocmlcvg.blob.core.windows.net
Does Water Evaporate Out Of Pool at Ronnie Hawk blog Why Does Water Evaporate Fast Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. What is evaporation and why does it occur? Water moves from the earth’s surface to the. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When liquid water meets dry. Why Does Water Evaporate Fast.
From fyocmlcvg.blob.core.windows.net
Does Water Evaporate Out Of Pool at Ronnie Hawk blog Why Does Water Evaporate Fast Evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Changes in pressure, more so than temperature, strongly. Why Does Water Evaporate Fast.
From materialcampusenterate.z14.web.core.windows.net
Does Salt Water Evaporate Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Changes in pressure, more so than temperature,. Why Does Water Evaporate Fast.
From brainly.in
how does evaporation cause cooling?? Brainly.in Why Does Water Evaporate Fast The level of humidity in the air also plays a role in the process of evaporation. When liquid water meets dry air, it is not in equilibrium; Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the. Why Does Water Evaporate Fast.
From waterdefense.org
Does Chlorine Evaporate from Tap Water? (Quick Answer!) Why Does Water Evaporate Fast Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation hasn’t been studied in detail at. Evaporation is the process that changes liquid water to gaseous water (water vapor). When liquid water meets dry air, it is not in equilibrium; What is evaporation and why does it. Why Does Water Evaporate Fast.
From www.scienceabc.com
Why Water In Lakes Doesn't Just Evaporate Or Seep Into The Ground? Why Does Water Evaporate Fast Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. Evaporation is the process that changes liquid water to gaseous water (water vapor). When the surface is exposed to sunlight, some molecules gain. Why Does Water Evaporate Fast.
From www.youtube.com
Why does ocean water evaporate even though it does not reach a boiling Why Does Water Evaporate Fast The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water moves from the earth’s surface to the. Liquid water is made up of molecules of h 2 o attracted to one another by. Why Does Water Evaporate Fast.
From www.slideserve.com
PPT Splish! Splash! WATER! PowerPoint Presentation, free download Why Does Water Evaporate Fast When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. What is evaporation and why does it occur? Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to gas, researchers show. Liquid water is made up of molecules of h 2 o attracted to. Why Does Water Evaporate Fast.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Why Does Water Evaporate Fast Evaporation hasn’t been studied in detail at. When clothes are hung on a laundry line, even though the ambient temperature is below the boiling point of water, water evaporates. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The level of humidity in the air also plays a role in the process. Why Does Water Evaporate Fast.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Why Does Water Evaporate Fast When liquid water meets dry air, it is not in equilibrium; Water moves from the earth’s surface to the. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to. Why Does Water Evaporate Fast.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo Why Does Water Evaporate Fast The pressure pushing down on the water makes it difficult for water to escape into the atmosphere as vapor. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to. Why Does Water Evaporate Fast.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The level of humidity in the air also plays a role in the process of evaporation.. Why Does Water Evaporate Fast.
From www.bol.com
Why Does Water Evaporate?, Rob Moore 9780521743563 Boeken Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When liquid water meets dry air, it is not in equilibrium; Water moves from the earth’s surface to the. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Evaporation hasn’t. Why Does Water Evaporate Fast.
From sciencing.com
Fast Ways to Make Water Evaporate Sciencing Why Does Water Evaporate Fast Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation hasn’t been studied in detail at. What is evaporation and why does it occur? Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When. Why Does Water Evaporate Fast.
From giocpjsdn.blob.core.windows.net
How Fast Does Water Evaporate In A Pond at Dennis Jones blog Why Does Water Evaporate Fast Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When liquid water meets dry air, it is not in equilibrium; What is evaporation and why does it occur? Evaporation hasn’t been studied in detail at. Changes in pressure, more so than temperature, strongly influence how quickly liquids turn to. Why Does Water Evaporate Fast.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Does Water Evaporate Fast Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Evaporation hasn’t been studied in detail at. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When the surface is exposed to sunlight, some molecules. Why Does Water Evaporate Fast.