What Is The Ph Of Water After Boiling . hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. In most cases, pure water will. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. However, if the temperature of the water increases by 167°f,. at room temperature (25 degrees celsius) the ph of pure water is 7. If you increase the temperature to 100. Particles in the water can change the ph of the water, and most.
from complete-water.com
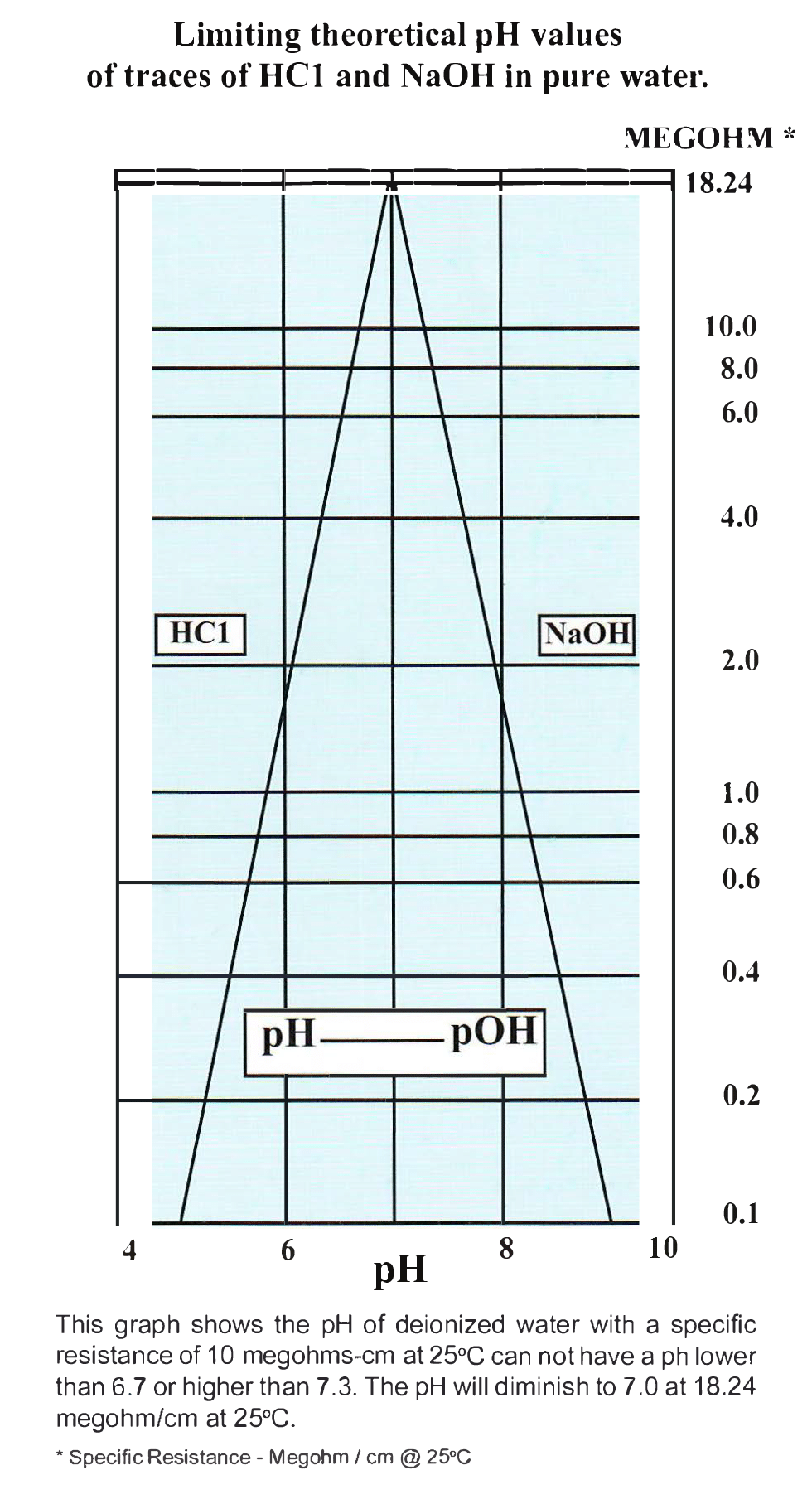
at room temperature (25 degrees celsius) the ph of pure water is 7. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. If you increase the temperature to 100. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. In most cases, pure water will. However, if the temperature of the water increases by 167°f,. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. Particles in the water can change the ph of the water, and most.
What is the pH of DI Water? Complete Water Solutions
What Is The Ph Of Water After Boiling If you increase the temperature to 100. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. Particles in the water can change the ph of the water, and most. If you increase the temperature to 100. at room temperature (25 degrees celsius) the ph of pure water is 7. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. In most cases, pure water will. However, if the temperature of the water increases by 167°f,. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature.
From mywaterearth.com
Safe pH Level for Drinking Water MyWaterEarth&Sky What Is The Ph Of Water After Boiling when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. at room temperature (25 degrees celsius) the ph of pure water is 7. If you increase the temperature to 100. In most cases, pure water will. However, if the temperature of the water increases by 167°f,. at 100°c, the ph of. What Is The Ph Of Water After Boiling.
From chemguru.sg
How to Calculate pH of Water What Is The Ph Of Water After Boiling at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. at room temperature (25 degrees celsius) the ph of pure water is 7. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. However, if the temperature of the water increases. What Is The Ph Of Water After Boiling.
From news.regenerativemedgroup.com
The pH of water What to know Regenerative Medical Group What Is The Ph Of Water After Boiling If you increase the temperature to 100. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. However, if the temperature of the water increases by 167°f,. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling.. What Is The Ph Of Water After Boiling.
From complete-water.com
What is the pH of DI Water? Complete Water Solutions What Is The Ph Of Water After Boiling at room temperature (25 degrees celsius) the ph of pure water is 7. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. in its purest form, water. What Is The Ph Of Water After Boiling.
From watertechadvice.com
pH of Water Everything You Need To Know What Is The Ph Of Water After Boiling However, if the temperature of the water increases by 167°f,. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral. What Is The Ph Of Water After Boiling.
From perfect-hydration.com
Drinking Water pH Explained What Should Be the Ideal pH? Perfect What Is The Ph Of Water After Boiling If you increase the temperature to 100. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. hence the ph of pure water is always neutral, with a value of. What Is The Ph Of Water After Boiling.
From chemguru.sg
How to Calculate pH of Water What Is The Ph Of Water After Boiling in its purest form, water has a ph of 7, which is at the exact center of the ph scale. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Particles in the water can change the ph of the water, and most. since the ph value. What Is The Ph Of Water After Boiling.
From exogzettv.blob.core.windows.net
What Is Ph Of Distilled Water At Normal Temperature at Guy Hobbs blog What Is The Ph Of Water After Boiling since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. Particles in the water can change the ph of the water, and most. in its purest form, water has. What Is The Ph Of Water After Boiling.
From zanyplantworld.com
PH Water Levels For The Plants Definition, Benefits, And Effects What Is The Ph Of Water After Boiling at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Particles in the water can change the ph of the water, and most. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. In most cases, pure. What Is The Ph Of Water After Boiling.
From skfelixer.com
The pH Value Of Purified Water — All You Need To Know SKF Elixer What Is The Ph Of Water After Boiling when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. at room temperature (25 degrees celsius) the ph of pure water is 7. However, if the temperature of the water increases by 167°f,. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor. What Is The Ph Of Water After Boiling.
From netsolwater.com
What is pH in water? How is pH level measured What Is The Ph Of Water After Boiling since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. In most cases, pure water will. when you’re measuring the ph of water, a 7.0 reading means that the. What Is The Ph Of Water After Boiling.
From upberi.com
What to Know about the pH of Drinking Water (2022) What Is The Ph Of Water After Boiling in its purest form, water has a ph of 7, which is at the exact center of the ph scale. However, if the temperature of the water increases by 167°f,. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. In most cases, pure water will. since the ph value. What Is The Ph Of Water After Boiling.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Water After Boiling since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. at room temperature (25 degrees celsius) the ph of pure water is 7. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. In most cases, pure water will. Particles. What Is The Ph Of Water After Boiling.
From www.bbc.co.uk
What is the pH scale and what does it measure? BBC Bitesize What Is The Ph Of Water After Boiling in its purest form, water has a ph of 7, which is at the exact center of the ph scale. If you increase the temperature to 100. at room temperature (25 degrees celsius) the ph of pure water is 7. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at. What Is The Ph Of Water After Boiling.
From www.plumbingsupply.com
Water pH and Your Plumbing What Is The Ph Of Water After Boiling since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. in its purest form, water has a ph of 7, which is at the exact center of the ph scale.. What Is The Ph Of Water After Boiling.
From inspectapedia.com
WHO pH for drinking water, too high pH or too low pH acidic What Is The Ph Of Water After Boiling hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. However, if the temperature of the water increases by 167°f,. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. at room temperature (25 degrees celsius) the ph of. What Is The Ph Of Water After Boiling.
From beerandgardeningjournal.com
The Easy Way To Hit The Proper Boil pH What Is The Ph Of Water After Boiling hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. Particles in the water can change the ph of the water, and most. If you increase the temperature to 100.. What Is The Ph Of Water After Boiling.
From www.intec-america.com
Things You Must Know About pH Control and Drinking Water Treatment What Is The Ph Of Water After Boiling at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. Particles in the water can change the ph of the water, and most. in its purest form, water has a ph. What Is The Ph Of Water After Boiling.
From blog.jencoi.com
Water Quality 101 What Is pH in Water Testing? What Is The Ph Of Water After Boiling If you increase the temperature to 100. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. In most cases, pure water will. However, if the temperature of the water increases by 167°f,. Particles in the water can change the ph of the water, and most. when. What Is The Ph Of Water After Boiling.
From opentextbc.ca
Water Hardness and pH Understanding Ingredients for the Canadian Baker What Is The Ph Of Water After Boiling Particles in the water can change the ph of the water, and most. However, if the temperature of the water increases by 167°f,. In most cases, pure water will. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. when you’re measuring the ph of water, a. What Is The Ph Of Water After Boiling.
From www.waterev.com
What is the pH Of Distilled Water? What Is The Ph Of Water After Boiling If you increase the temperature to 100. Particles in the water can change the ph of the water, and most. at room temperature (25 degrees celsius) the ph of pure water is 7. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. However, if the temperature of. What Is The Ph Of Water After Boiling.
From www.curezone.org
Can you use baking soda to boost boiled water ph on a fast? at Fasting What Is The Ph Of Water After Boiling at room temperature (25 degrees celsius) the ph of pure water is 7. If you increase the temperature to 100. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. in its purest form, water has a ph of 7, which is at the exact center of. What Is The Ph Of Water After Boiling.
From www.survivopedia.com
How To Measure Water pH At Home Survivopedia What Is The Ph Of Water After Boiling at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. In most cases, pure water will. since the ph value is tied to the concentration of the. What Is The Ph Of Water After Boiling.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Water After Boiling at room temperature (25 degrees celsius) the ph of pure water is 7. In most cases, pure water will. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. Particles in the water can change the ph of the water, and most. If you increase the temperature. What Is The Ph Of Water After Boiling.
From upberi.com
What to Know about the pH of Drinking Water (2022) What Is The Ph Of Water After Boiling If you increase the temperature to 100. at room temperature (25 degrees celsius) the ph of pure water is 7. Particles in the water can change the ph of the water, and most. In most cases, pure water will. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic). What Is The Ph Of Water After Boiling.
From waterseer.org
What is pH in Water? Definition, Importance & Level Chart What Is The Ph Of Water After Boiling Particles in the water can change the ph of the water, and most. In most cases, pure water will. at room temperature (25 degrees celsius) the ph of pure water is 7. when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. since the ph value is tied to the concentration. What Is The Ph Of Water After Boiling.
From www.expii.com
What Is pH? — Definition & Overview Expii What Is The Ph Of Water After Boiling In most cases, pure water will. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. If you increase the temperature to 100. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. when you’re measuring. What Is The Ph Of Water After Boiling.
From www.dyln.co
Everything You Need To Know About Alkaline Water DYLN What Is The Ph Of Water After Boiling hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of. What Is The Ph Of Water After Boiling.
From ceuqlrkm.blob.core.windows.net
How Long After Boiling Water To Cool at Catherine Thornhill blog What Is The Ph Of Water After Boiling Particles in the water can change the ph of the water, and most. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. If you increase the temperature to 100. However, if the temperature of the water increases by 167°f,. at room temperature (25 degrees celsius) the. What Is The Ph Of Water After Boiling.
From blog.havells.com
Right pH Level in Drinking Water How Essential? Havells India Blog What Is The Ph Of Water After Boiling when you’re measuring the ph of water, a 7.0 reading means that the water is neutral. at room temperature (25 degrees celsius) the ph of pure water is 7. However, if the temperature of the water increases by 167°f,. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this. What Is The Ph Of Water After Boiling.
From upberi.com
What to Know about the pH of Drinking Water (2022) What Is The Ph Of Water After Boiling In most cases, pure water will. at room temperature (25 degrees celsius) the ph of pure water is 7. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. If you increase the temperature to 100. Particles in the water can change the ph of the water, and. What Is The Ph Of Water After Boiling.
From exoflhije.blob.core.windows.net
The Ph Value Of The Water Used In Boiler Is at Ernesto Barrera blog What Is The Ph Of Water After Boiling in its purest form, water has a ph of 7, which is at the exact center of the ph scale. since the ph value is tied to the concentration of the $\ce{h+}$, a neutral probe (neither acidic, nor basic) of boiling. hence the ph of pure water is always neutral, with a value of 7.0 at room. What Is The Ph Of Water After Boiling.
From www.iliv.co.in
Does the pH Level of Alkaline Water Remain Constant after Boiling or What Is The Ph Of Water After Boiling However, if the temperature of the water increases by 167°f,. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. If you increase the temperature to 100. in its purest form, water has a ph of 7, which is at the exact center of the ph scale. . What Is The Ph Of Water After Boiling.
From aquaclearws.com
Why Is The pH of Water Important? Aqua Clear Water Systems What Is The Ph Of Water After Boiling at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Particles in the water can change the ph of the water, and most. hence the ph of pure water is always neutral, with a value of 7.0 at room temperature. However, if the temperature of the water increases. What Is The Ph Of Water After Boiling.
From netsolwater.com
What is pH in water, and what is pH in wastewater? Netsol Water What Is The Ph Of Water After Boiling in its purest form, water has a ph of 7, which is at the exact center of the ph scale. In most cases, pure water will. at 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Particles in the water can change the ph of the water, and. What Is The Ph Of Water After Boiling.