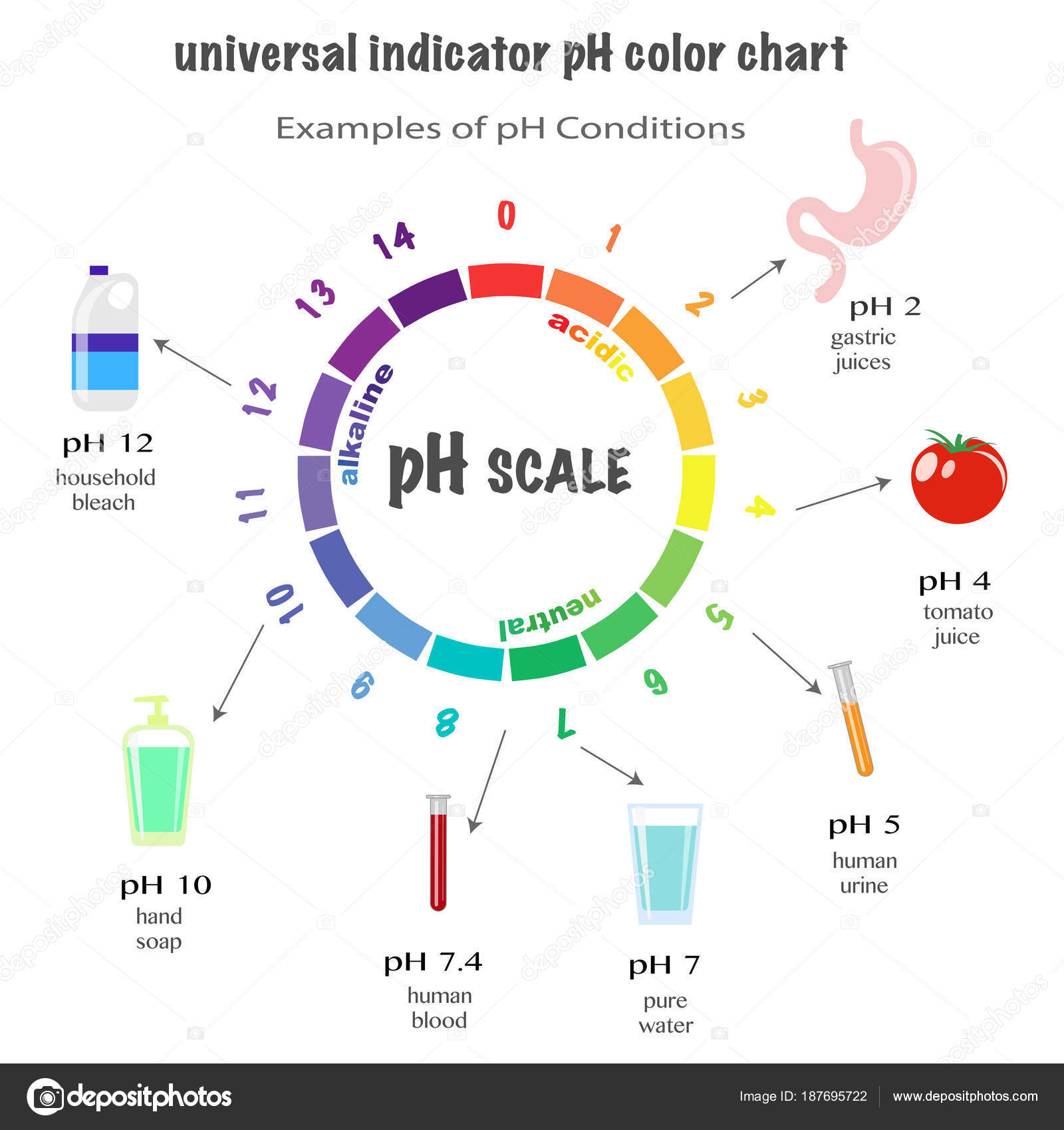
Base Examples Ph . To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A neutral ph value (neither an acid nor a base) is 7. Explain the ph scale, and convert ph and concentration of. A ph less than 7 indicates acidity, while. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Substances with a ph greater. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Acidity and alkalinity are measured with a logarithmic scale called ph. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. A strongly acidic solution can have one hundred million million, or one hundred trillion. Bases have a ph value higher than 7, with common examples including ammonia and lye. Give the names and formulas of some strong acids and bases. In simple terms, bases are the chemical opposites of acids.
from ar.inspiredpencil.com
A ph less than 7 indicates acidity, while. A strongly acidic solution can have one hundred million million, or one hundred trillion. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Bases have a ph value higher than 7, with common examples including ammonia and lye. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Give the names and formulas of some strong acids and bases. Acidity and alkalinity are measured with a logarithmic scale called ph. Explain the ph scale, and convert ph and concentration of. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. Substances with a ph greater.
Ph Level Of Examples And Their Bases
Base Examples Ph Explain the ph scale, and convert ph and concentration of. Bases have a ph value higher than 7, with common examples including ammonia and lye. Acidity and alkalinity are measured with a logarithmic scale called ph. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A ph less than 7 indicates acidity, while. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. In simple terms, bases are the chemical opposites of acids. Explain the ph scale, and convert ph and concentration of. Substances with a ph greater. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. A neutral ph value (neither an acid nor a base) is 7. Give the names and formulas of some strong acids and bases. A strongly acidic solution can have one hundred million million, or one hundred trillion. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Generally, the value of ph of acids and bases are used to quantitatively determine their strength.
From www.ck12.org
Water, Acids, and Bases CK12 Foundation Base Examples Ph The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Acidity and alkalinity are measured with a logarithmic scale called ph. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. A strongly acidic. Base Examples Ph.
From www.alamy.com
scale of ph value for acid and alkaline solutions, infographic acid Base Examples Ph Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Bases have a ph value higher than 7, with common examples including ammonia and lye. The ph scale, ranging from 0 to 14, measures the acidity. Base Examples Ph.
From www.preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories Base Examples Ph Generally, the value of ph of acids and bases are used to quantitatively determine their strength. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. In simple terms, bases are the chemical opposites of acids. Substances with a ph greater. Give the names and formulas of some strong acids and bases. Ph is a measure of. Base Examples Ph.
From content.byui.edu
Acids, Bases, pH and Buffers Base Examples Ph Give the names and formulas of some strong acids and bases. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Substances with a ph greater. A ph less than 7 indicates acidity, while. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer.. Base Examples Ph.
From www.fiquipedia.es
Recursos ácidos y bases FiQuiPedia Base Examples Ph In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Acidity and alkalinity are measured with a logarithmic scale called ph. A ph less than 7 indicates acidity, while. A strongly acidic solution can have one hundred million million, or one hundred trillion. Substances with a ph greater. A neutral ph value (neither an acid nor a. Base Examples Ph.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Base Examples Ph A ph less than 7 indicates acidity, while. Bases have a ph value higher than 7, with common examples including ammonia and lye. Acidity and alkalinity are measured with a logarithmic scale called ph. A neutral ph value (neither an acid nor a base) is 7. Generally, the value of ph of acids and bases are used to quantitatively determine. Base Examples Ph.
From www.narodnatribuna.info
Ph Scale Acids And Bases Examples Base Examples Ph A neutral ph value (neither an acid nor a base) is 7. A ph less than 7 indicates acidity, while. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. In simple terms, bases are the chemical opposites of acids. Generally, the value of ph of acids and bases are used to. Base Examples Ph.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG Base Examples Ph Give the names and formulas of some strong acids and bases. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. A neutral ph value (neither an acid nor a base) is 7. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. A ph less than. Base Examples Ph.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler Base Examples Ph Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Bases have a ph value higher than 7, with common examples including ammonia and lye. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. Substances with a ph greater. In chemistry, ph is a number. Base Examples Ph.
From sciencenotes.org
How to Calculate pH Formula and Examples Base Examples Ph The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. In simple terms, bases are the chemical opposites of acids. A strongly acidic solution can have one hundred million million, or one hundred trillion. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Explain the ph scale, and convert. Base Examples Ph.
From www.slideserve.com
PPT Reactions of acids and bases… PowerPoint Presentation, free Base Examples Ph Bases have a ph value higher than 7, with common examples including ammonia and lye. Substances with a ph greater. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Acidity and alkalinity are measured with a. Base Examples Ph.
From depositphotos.com
Ph scale. infographic acidbase balance. scale for chemical analysis Base Examples Ph Give the names and formulas of some strong acids and bases. A ph less than 7 indicates acidity, while. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. In chemistry, ph is a number that acidity. Base Examples Ph.
From pinterest.com
Common examples of acids and bases & a great explanation of how the pH Base Examples Ph A strongly acidic solution can have one hundred million million, or one hundred trillion. Substances with a ph greater. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Explain the ph scale, and convert. Base Examples Ph.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog Base Examples Ph Generally, the value of ph of acids and bases are used to quantitatively determine their strength. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. A ph less than 7 indicates acidity, while. Acidity and alkalinity are measured with a logarithmic scale called ph. In simple terms, bases are the chemical opposites of acids. A strongly. Base Examples Ph.
From sciencenotes.org
The pH Scale of Common Chemicals Base Examples Ph Substances with a ph greater. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. A ph less than 7 indicates acidity, while. To calculate ph, take the log of the hydrogen ion concentration and. Base Examples Ph.
From thebaseisunderasalt.weebly.com
pH Scale THe base is under a salt Base Examples Ph In simple terms, bases are the chemical opposites of acids. Give the names and formulas of some strong acids and bases. Acidity and alkalinity are measured with a logarithmic scale called ph. Substances with a ph greater. Bases have a ph value higher than 7, with common examples including ammonia and lye. A strongly acidic solution can have one hundred. Base Examples Ph.
From depositphotos.com
Scale of ph value for acid and alkaline solutions, infographic acid Base Examples Ph Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Explain the ph scale, and convert ph and concentration of. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Substances with a ph greater. The ph scale, ranging from 0 to 14, measures the acidity. Base Examples Ph.
From www.worksheetsplanet.com
Acid and Bases Differences Base Examples Ph Substances with a ph greater. A ph less than 7 indicates acidity, while. A strongly acidic solution can have one hundred million million, or one hundred trillion. Explain the ph scale, and convert ph and concentration of. Bases have a ph value higher than 7, with common examples including ammonia and lye. To calculate ph, take the log of the. Base Examples Ph.
From study.com
Acidic, Basic & Neutral Solutions Determining pH Video & Lesson Base Examples Ph To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. Bases have a ph value higher than 7, with common examples including ammonia and lye. Acidity and alkalinity are measured with a logarithmic scale called ph. A neutral ph value (neither an acid nor a base) is 7. Explain the ph scale,. Base Examples Ph.
From secondaryscience4all.wordpress.com
Acids and bases Secondary Science 4 All Base Examples Ph Bases have a ph value higher than 7, with common examples including ammonia and lye. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. A strongly acidic solution can have one hundred million million, or. Base Examples Ph.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline Base Examples Ph In simple terms, bases are the chemical opposites of acids. Give the names and formulas of some strong acids and bases. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Acidity and alkalinity are measured with a logarithmic scale called ph. To calculate ph, take the log of the hydrogen ion concentration and. Base Examples Ph.
From ar.inspiredpencil.com
Ph Level Of Examples And Their Bases Base Examples Ph Generally, the value of ph of acids and bases are used to quantitatively determine their strength. A neutral ph value (neither an acid nor a base) is 7. A ph less than 7 indicates acidity, while. A strongly acidic solution can have one hundred million million, or one hundred trillion. Substances with a ph greater. The ph scale, ranging from. Base Examples Ph.
From www.dreamstime.com
Acids, Neutral and Bases Substances Scale with Examples Outline Diagram Base Examples Ph The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Give the names and formulas of some strong acids and bases. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Explain the ph scale, and convert ph and concentration of. Substances with a ph greater. In. Base Examples Ph.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii Base Examples Ph A strongly acidic solution can have one hundred million million, or one hundred trillion. Give the names and formulas of some strong acids and bases. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. A ph less than 7 indicates acidity, while. Substances with a ph greater. The ph scale, ranging from 0. Base Examples Ph.
From acidsandbasesrios.weebly.com
pH Acids and Bases Base Examples Ph To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. Explain the ph scale, and convert ph and concentration of. Acidity and alkalinity are measured with a logarithmic scale called ph. Bases have a ph value higher than 7, with common examples including ammonia and lye. A ph less than 7 indicates. Base Examples Ph.
From kidspressmagazine.com
Acids and Bases Base Examples Ph Give the names and formulas of some strong acids and bases. Explain the ph scale, and convert ph and concentration of. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Acidity and alkalinity are measured with a logarithmic scale called ph. Generally, the value of ph of acids and bases are used to quantitatively determine their. Base Examples Ph.
From sciencenotes.org
Facts About Acids and Bases Base Examples Ph The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A ph less than 7 indicates acidity, while. Give the names and formulas of some strong acids and bases. Explain the ph scale, and convert. Base Examples Ph.
From www.dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators Base Examples Ph To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A neutral ph value (neither an acid nor a base) is 7. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Substances with a ph greater. In simple terms, bases are the chemical. Base Examples Ph.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub Base Examples Ph In simple terms, bases are the chemical opposites of acids. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. A ph less than 7 indicates acidity, while. Give the names and formulas of some strong acids and bases. To calculate ph, take the log of the hydrogen ion concentration and change the. Base Examples Ph.
From www.youtube.com
How to Calculate the pH of Strong Bases Practice Problems, Examples Base Examples Ph Explain the ph scale, and convert ph and concentration of. Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Acidity and alkalinity are measured with a logarithmic scale called ph. To calculate ph, take the log of the. Base Examples Ph.
From www.slideserve.com
PPT Acids, Bases, and pH Scales PowerPoint Presentation, free Base Examples Ph Ph is a measure of how acidic or basic a chemical is when it's in aqueous (water) solution. Substances with a ph greater. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Bases have a ph value higher than 7, with common examples including ammonia and lye. Generally, the value of ph of acids and bases. Base Examples Ph.
From www.ck12.org
Acids and Bases CK12 Foundation Base Examples Ph In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Bases have a ph value higher than 7, with common examples including ammonia and lye. Substances with a ph greater. A ph less than 7 indicates acidity, while. Generally, the value of ph of acids and bases are used to quantitatively determine their strength. Acidity and alkalinity. Base Examples Ph.
From www.narodnatribuna.info
Ph Scale Acids And Bases Examples Base Examples Ph To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. Give the names and formulas of some strong acids and bases. In chemistry, ph is a number that acidity or basicity (alkalinity) of an. Substances with a ph greater. A neutral ph value (neither an acid nor a base) is 7. Explain. Base Examples Ph.
From mchsscience.weebly.com
Acids and Bases MCHS Science Base Examples Ph Explain the ph scale, and convert ph and concentration of. To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A ph less than 7 indicates acidity, while. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. In chemistry, ph is a number that. Base Examples Ph.
From byjus.com
pH Chemistry (Acids & Bases) Definition, Calculating pH Value, Videos Base Examples Ph To calculate ph, take the log of the hydrogen ion concentration and change the sign of the answer. A strongly acidic solution can have one hundred million million, or one hundred trillion. A neutral ph value (neither an acid nor a base) is 7. Substances with a ph greater. A ph less than 7 indicates acidity, while. In simple terms,. Base Examples Ph.