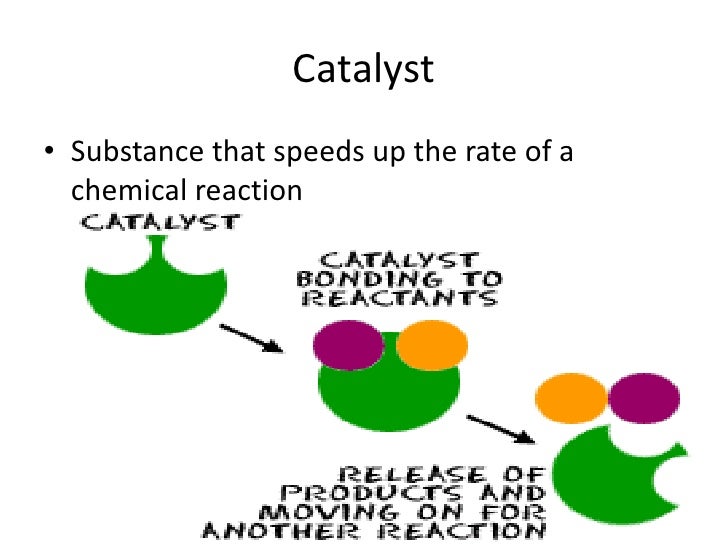
Catalyst Energy Definition . Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts allow a reaction to. It is not consumed by the process. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst lowers the activation energy of a reaction, increasing its rate. List examples of catalysis in natural. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In chemistry and biology, a catalyst is a substance the increases. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is some material that speeds up chemical reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts participate in a chemical reaction and increase its rate. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts work by providing an alternative.
from www.slideshare.net
A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; Catalysts work by providing an alternative. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is some material that speeds up chemical reactions.
Biology 2.4
Catalyst Energy Definition In chemistry and biology, a catalyst is a substance the increases. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts work by providing an alternative. It is not consumed by the process. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is some material that speeds up chemical reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases. Catalysts participate in a chemical reaction and increase its rate. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalysts allow a reaction to. List examples of catalysis in natural. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Energy Definition A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is some material that speeds up chemical. Catalyst Energy Definition.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Energy Definition With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; In chemistry and biology, a catalyst is a substance. Catalyst Energy Definition.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Energy Definition A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts allow a reaction to. A catalyst is some material that speeds up chemical reactions. It is not consumed by the process. Explain the function of a catalyst in terms of reaction. Catalyst Energy Definition.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Energy Definition With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In chemistry and biology, a catalyst is a substance the increases. A catalyst is some material that speeds up chemical reactions. Catalysts work by providing an alternative. A catalyst, in turn, is a substance that is not consumed by the. Catalyst Energy Definition.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Energy Definition With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. List examples of catalysis in natural. Catalysts work by providing an alternative. They do not appear in the reaction’s net equation and are not consumed during the reaction. It is not consumed by the process. Catalysis is defined as increasing. Catalyst Energy Definition.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalyst Energy Definition List examples of catalysis in natural. Catalysts allow a reaction to. In chemistry and biology, a catalyst is a substance the increases. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. They do not appear in the reaction’s net equation and are not consumed during the reaction. A. Catalyst Energy Definition.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Energy Definition Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in natural. Catalysts work by providing an alternative. In chemistry and biology, a catalyst is a substance the increases. It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts allow a reaction to. A catalyst is. Catalyst Energy Definition.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst Energy Definition In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts allow a reaction to. A catalyst is some material that speeds up chemical reactions. In chemistry and biology, a catalyst is a substance the increases. It. Catalyst Energy Definition.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalyst Energy Definition It is not consumed by the process. Catalysts participate in a chemical reaction and increase its rate. In chemistry and biology, a catalyst is a substance the increases. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. With a helping hand from a catalyst, molecules that might take years to interact can now. Catalyst Energy Definition.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Energy Definition It is not consumed by the process. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst is some material that speeds up chemical reactions. Catalysts. Catalyst Energy Definition.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Energy Definition Catalysts allow a reaction to. In chemistry and biology, a catalyst is a substance the increases. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural. In chemistry, a catalyst is a substance that increases the rate of a. Catalyst Energy Definition.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalyst Energy Definition Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst lowers the activation energy of a reaction, increasing its rate. With a helping hand from a catalyst, molecules that might take years to interact can now do so. Catalyst Energy Definition.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Energy Definition Catalysts participate in a chemical reaction and increase its rate. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. List examples of catalysis in natural. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is. Catalyst Energy Definition.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Energy Definition A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. It is not consumed by the process. List examples of catalysis in natural. Catalysts allow a reaction to. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. With a helping hand from. Catalyst Energy Definition.
From ar.inspiredpencil.com
Catalyst Chemistry Catalyst Energy Definition With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It is not consumed by the process. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is some material that speeds up chemical reactions. Catalysts work by providing an alternative. A catalyst. Catalyst Energy Definition.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Energy Definition A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases. List examples of catalysis in natural. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalysts participate in a chemical reaction and increase its rate. Catalysts work by providing an alternative.. Catalyst Energy Definition.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Energy Definition List examples of catalysis in natural. In chemistry and biology, a catalyst is a substance the increases. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is some material that speeds up chemical reactions. Catalysts work by providing an alternative. They do not appear in the reaction’s. Catalyst Energy Definition.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Energy Definition Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to. Catalysts work by providing an alternative. Explain the function of a catalyst in terms of. Catalyst Energy Definition.
From slideplayer.com
CATALYST “Energy cannot be created or destroyed but can be converted Catalyst Energy Definition In chemistry and biology, a catalyst is a substance the increases. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. In chemistry, a catalyst is a substance that increases the rate of a. Catalyst Energy Definition.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Energy Definition Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalysts participate in a chemical reaction and increase its rate. It is not consumed. Catalyst Energy Definition.
From www.catalystseurope.org
What are catalysts? Catalyst Energy Definition A catalyst is some material that speeds up chemical reactions. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysis is defined as increasing the rate of a chemical reaction by. Catalyst Energy Definition.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Energy Definition List examples of catalysis in natural. A catalyst is some material that speeds up chemical reactions. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It is not consumed. Catalyst Energy Definition.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Energy Definition Catalysts allow a reaction to. It is not consumed by the process. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain the. Catalyst Energy Definition.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Energy Definition A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural. Catalysts allow a reaction to. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts participate in a chemical reaction and increase its rate. Catalysis is. Catalyst Energy Definition.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Energy Definition Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts allow a reaction to. A catalyst is some material that speeds up chemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and potential energy. Catalyst Energy Definition.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID Catalyst Energy Definition In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction. Catalyst Energy Definition.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Energy Definition Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the. Catalyst Energy Definition.
From www.pnnl.gov
Catalysis PNNL Catalyst Energy Definition It is not consumed by the process. List examples of catalysis in natural. Catalysts work by providing an alternative. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams; In chemistry and biology, a catalyst is a substance the increases. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst.. Catalyst Energy Definition.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst Energy Definition A catalyst is some material that speeds up chemical reactions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. In chemistry and biology, a catalyst is a substance the increases. Catalysts allow. Catalyst Energy Definition.
From www.thoughtco.com
Catalyst Definition as Used in Chemistry Catalyst Energy Definition A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts participate in a chemical reaction and increase its rate. Catalysts work by providing an alternative. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. List examples of catalysis in natural. A catalyst is a chemical. Catalyst Energy Definition.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Energy Definition In chemistry and biology, a catalyst is a substance the increases. It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts participate in a chemical reaction and increase its rate. Catalysts work by providing an alternative. A catalyst is some material that speeds up chemical reactions. In chemistry, a catalyst. Catalyst Energy Definition.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalyst Energy Definition Catalysts work by providing an alternative. They do not appear in the reaction’s net equation and are not consumed during the reaction. It is not consumed by the process. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst, in turn, is a substance that is not consumed. Catalyst Energy Definition.
From www.slideshare.net
Biology 2.4 Catalyst Energy Definition A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. In chemistry and biology, a catalyst is a substance the increases. A catalyst is some material that speeds up chemical reactions. They do not appear in the reaction’s net equation and are not consumed during. Catalyst Energy Definition.
From www.slideserve.com
PPT Section 24 & 25 “Chemical Reactions & Enzymes ” PowerPoint Catalyst Energy Definition A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In chemistry, a catalyst is a substance. Catalyst Energy Definition.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Energy Definition In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. In chemistry and biology, a catalyst is a substance the increases.. Catalyst Energy Definition.