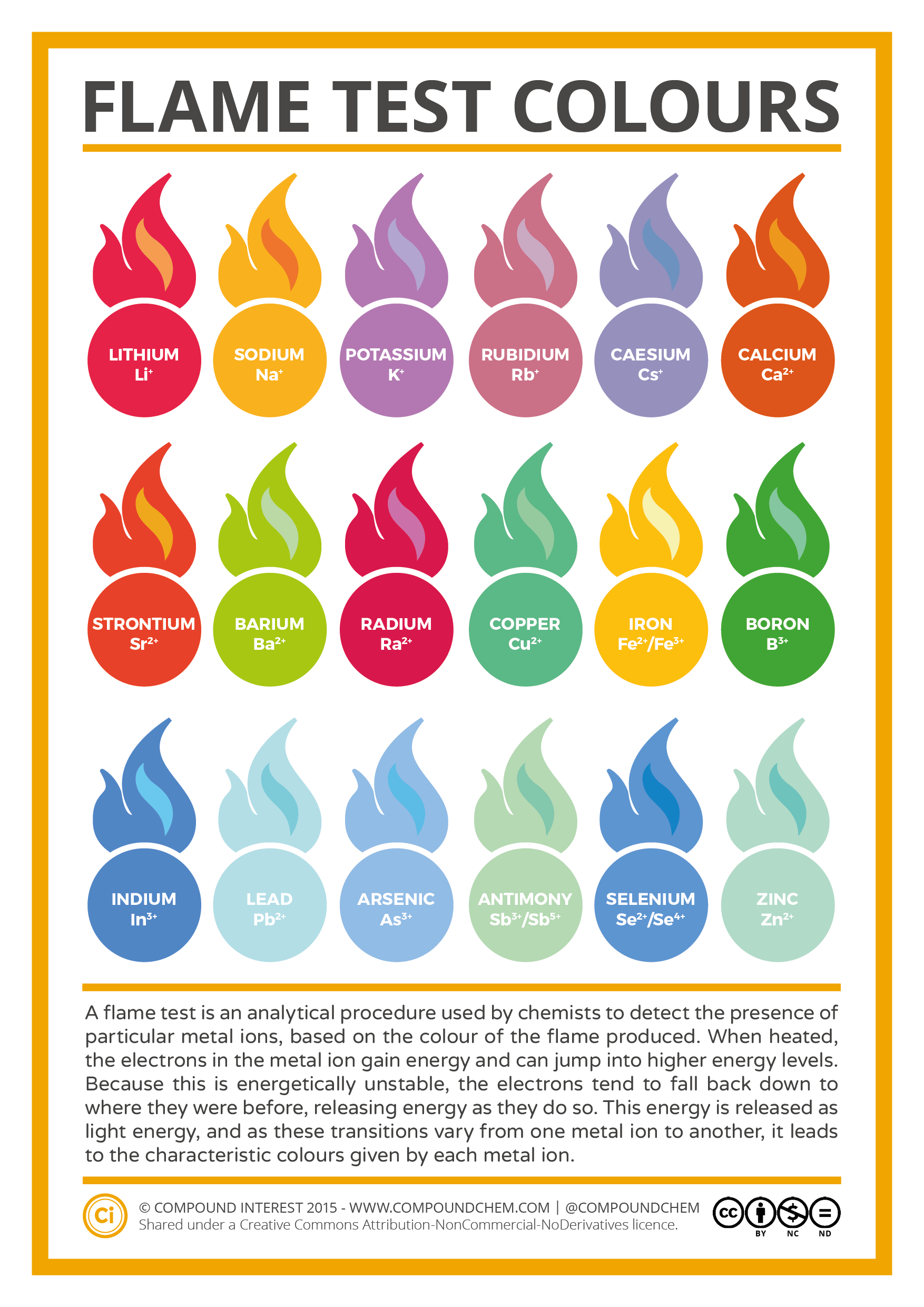
Copper Color When Burned . Different types of powdered metals and salts are sprinkled over a flame. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. A metal salt consists of a component cation (the metal) and an anion. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. This colour originates from the movement of electrons. To carry out a flame test: Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. The video shows the metals and salts in this order: An ionic compound occurs when a negative ion (an atom. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. The table shows the flame test colours for six common metal ions.
from www.chemeurope.com
To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. An ionic compound occurs when a negative ion (an atom. A metal salt consists of a component cation (the metal) and an anion. This colour originates from the movement of electrons. The table shows the flame test colours for six common metal ions. Different types of powdered metals and salts are sprinkled over a flame. To carry out a flame test: If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. The video shows the metals and salts in this order:
Metal Ion Flame Test Colours Chart
Copper Color When Burned An ionic compound occurs when a negative ion (an atom. Different types of powdered metals and salts are sprinkled over a flame. This colour originates from the movement of electrons. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. To carry out a flame test: A metal salt consists of a component cation (the metal) and an anion. The video shows the metals and salts in this order: An ionic compound occurs when a negative ion (an atom. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. The table shows the flame test colours for six common metal ions. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light.
From mucholderthen.tumblr.com
Science Visualized • Flame color of various elements as compounds in... Copper Color When Burned The table shows the flame test colours for six common metal ions. A metal salt consists of a component cation (the metal) and an anion. To carry out a flame test: An ionic compound occurs when a negative ion (an atom. The video shows the metals and salts in this order: Show the flame colours of alkali metals, alkaline earth. Copper Color When Burned.
From www.chemedx.org
Colorful Copper Chemistry around the Campfire Chemical Education Xchange Copper Color When Burned To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. A metal salt consists of a component cation (the metal) and an anion. To carry out a flame test: If you have ever done chemistry experiments in the laboratory, you might have noticed that. Copper Color When Burned.
From www.housedigest.com
The Easiest Ways To Clean Burnt Copper Copper Color When Burned To carry out a flame test: To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. This colour originates from the movement of electrons. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. If you have. Copper Color When Burned.
From www.dreamstime.com
Sheet Metal Painted a Copper Color. Background or Texture Stock Photo Copper Color When Burned The table shows the flame test colours for six common metal ions. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. An ionic compound occurs when a negative ion (an atom. For example, copper (i) emits blue light during the flame test, while copper (ii). Copper Color When Burned.
From www.freepik.com
Premium AI Image Textured surface with copper color Copper Color When Burned This colour originates from the movement of electrons. To carry out a flame test: If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. Different types of powdered metals and salts are sprinkled over a flame. A metal salt consists of a component cation (the metal). Copper Color When Burned.
From hipfonts.com
burnt red orange HipFonts Copper Color When Burned An ionic compound occurs when a negative ion (an atom. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. To carry out a flame test: Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium. Copper Color When Burned.
From www.pinterest.com
copper texture Google Search Texture, Patterns in nature, Painting Copper Color When Burned Different types of powdered metals and salts are sprinkled over a flame. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. To carry out a flame test: For example, copper (i) emits blue light during the flame test, while copper. Copper Color When Burned.
From sciencenotes.org
How to Make Colored Fire at Home Copper Color When Burned Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. The video shows the metals and salts in this order: An ionic compound occurs when a negative ion (an atom. A metal salt consists of a component cation (the metal) and. Copper Color When Burned.
From www.youtube.com
Burning Copper (II) Chloride In Chemistry Iowa State YouTube Copper Color When Burned The table shows the flame test colours for six common metal ions. An ionic compound occurs when a negative ion (an atom. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. A metal salt consists of a component cation (the metal) and an anion. The. Copper Color When Burned.
From creativebooster.net
50+ Shades of Copper Color (Names, HEX, RGB, & CMYK Codes Copper Color When Burned To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. An ionic compound occurs when. Copper Color When Burned.
From colors.artyclick.com
Pale Copper Color ArtyClick Copper Color When Burned To carry out a flame test: Different types of powdered metals and salts are sprinkled over a flame. The table shows the flame test colours for six common metal ions. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. To. Copper Color When Burned.
From pixels.com
Copper Flame Test Photograph by Science Photo Library Pixels Copper Color When Burned For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. The video shows the metals and salts in this order: An ionic compound occurs when a negative. Copper Color When Burned.
From joiubmlbh.blob.core.windows.net
Light Burnt Orange Color Code at Cassandra Numbers blog Copper Color When Burned This colour originates from the movement of electrons. To carry out a flame test: An ionic compound occurs when a negative ion (an atom. The video shows the metals and salts in this order: Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and. Copper Color When Burned.
From in.eteachers.edu.vn
Aggregate 71+ copper colour hair in.eteachers Copper Color When Burned To carry out a flame test: To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. Different types of powdered metals and salts are sprinkled over a flame. The table shows the flame test colours for six common metal ions. The video shows the. Copper Color When Burned.
From pixels.com
Copper Flame Test Photograph by David Taylor/science Photo Library Pixels Copper Color When Burned An ionic compound occurs when a negative ion (an atom. Different types of powdered metals and salts are sprinkled over a flame. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. Show the flame colours of alkali metals, alkaline earth metals, and other. Copper Color When Burned.
From www.dreamstime.com
Reddish Copper Color Artwork. Stock Image Image of parchment Copper Color When Burned Different types of powdered metals and salts are sprinkled over a flame. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. To carry out a flame test: Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium. Copper Color When Burned.
From www.art-paints.com
Burnt Copper Flakes Airbrush Spray Paints 06074 Burnt Copper Paint Copper Color When Burned Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. This colour originates from the movement of. Copper Color When Burned.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Copper Color When Burned This colour originates from the movement of electrons. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. Different types of powdered metals and salts are sprinkled over a flame. For example, copper (i) emits blue light during the flame test,. Copper Color When Burned.
From www.chemeurope.com
Metal Ion Flame Test Colours Chart Copper Color When Burned If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. Different types of powdered metals and salts are sprinkled over a flame. A metal salt consists of a component cation (the metal) and an anion. Show the flame colours of alkali metals, alkaline earth metals, and. Copper Color When Burned.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Copper Color When Burned The table shows the flame test colours for six common metal ions. This colour originates from the movement of electrons. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour. Copper Color When Burned.
From www.deviantart.com
Burnished Copper by AnnaKirsten on DeviantArt Copper Color When Burned For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. An ionic compound occurs when a negative ion (an atom. This colour originates from the movement of electrons. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. Show. Copper Color When Burned.
From loganharvesthome.blogspot.com
17 Best Pictures Auburn Hair Copper Highlights 50 Auburn Hair Color Copper Color When Burned The table shows the flame test colours for six common metal ions. An ionic compound occurs when a negative ion (an atom. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. This colour originates from the movement of electrons. The video shows the metals and salts in this order: If you have. Copper Color When Burned.
From www.livescience.com
Facts About Copper Live Science Copper Color When Burned To carry out a flame test: To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. The table shows the flame test colours for six common metal ions. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely. Copper Color When Burned.
From stock.adobe.com
Copper colored wall texture background with textures of different Copper Color When Burned Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. To carry out a flame test: The video shows the metals and salts in this order: This colour originates from the movement of electrons. To carry out the flame tests, a. Copper Color When Burned.
From www.pinterest.ca
50+ Shades of Copper Color (Names, HEX, RGB, & CMYK Codes) Copper Copper Color When Burned A metal salt consists of a component cation (the metal) and an anion. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. An ionic compound occurs when a negative ion (an atom. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium. Copper Color When Burned.
From www.alamy.com
Copper colored background with textures of different shades of copper Copper Color When Burned To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. This colour originates from the movement of electrons. The table shows the flame test colours for six common metal ions. For example, copper (i) emits blue light during the flame test, while copper (ii). Copper Color When Burned.
From artyclick.com
Copper Color ArtyClick Copper Color When Burned If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. The table shows the flame test colours for six common metal ions. Different types of powdered metals and salts are sprinkled over a flame. A metal salt consists of a component cation (the metal) and an. Copper Color When Burned.
From fireplacetips.com
Why is Fire Blue (& Is It Hotter)? Answered Fireplace Tips Copper Color When Burned An ionic compound occurs when a negative ion (an atom. A metal salt consists of a component cation (the metal) and an anion. If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. For example, copper (i) emits blue light during the flame test, while copper. Copper Color When Burned.
From www.thoughtco.com
How To Make Green Flames Using Copper Sulfate Copper Color When Burned Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. To carry out the flame tests, a small amount of the compound being tested will be held in a flame and the colour given off observed. For example, copper (i) emits. Copper Color When Burned.
From www.alamy.com
Different colored flames of burning salts. Potassium permangate, copper Copper Color When Burned The table shows the flame test colours for six common metal ions. An ionic compound occurs when a negative ion (an atom. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate and ethanol through a. To carry out a flame test: Different types of. Copper Color When Burned.
From hairstylehub.com
30 Copper Hair Color Ideas Copper Color When Burned If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride,. Copper Color When Burned.
From www.dreamstime.com
Sheet Metal Painted a Copper Color. Background or Texture Stock Image Copper Color When Burned For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. This colour originates from the movement of electrons. The video shows the metals and salts in this order: Show the flame colours of alkali metals, alkaline earth metals, and other metal salts by safely spraying sodium chloride, potassium chloride, lithium chloride, copper sulfate. Copper Color When Burned.
From www.pinterest.com
Cosmopolitan UK’s edit of the best copper hair styles on Instagram now Copper Color When Burned If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. This colour originates from the movement of electrons. For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. Show the flame colours of alkali metals, alkaline earth metals, and. Copper Color When Burned.
From www.pinterest.com
Pin on Science Copper Color When Burned For example, copper (i) emits blue light during the flame test, while copper (ii) emits green light. The video shows the metals and salts in this order: A metal salt consists of a component cation (the metal) and an anion. An ionic compound occurs when a negative ion (an atom. Different types of powdered metals and salts are sprinkled over. Copper Color When Burned.
From howigotjob.com
What Colors Make Copper? How to make copper color? Copper Color When Burned If you have ever done chemistry experiments in the laboratory, you might have noticed that when metals are heated, the flame sometimes changes color. An ionic compound occurs when a negative ion (an atom. To carry out a flame test: Different types of powdered metals and salts are sprinkled over a flame. A metal salt consists of a component cation. Copper Color When Burned.