How Many Atoms Are In A Centimeter . The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Divide this by the mass of an atom to find, approximately, the number of atoms. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Today, they agree that atoms have a. Scientists’ ideas about atoms have changed over time. How to calculate the number of atoms in a sample. Equivalently, first find the number of atoms in $1$ cubic. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Converting both units to meters and using scientific. To calculate the number of atoms in a.
from www.chegg.com
Today, they agree that atoms have a. Converting both units to meters and using scientific. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Scientists’ ideas about atoms have changed over time. To calculate the number of atoms in a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Divide this by the mass of an atom to find, approximately, the number of atoms. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. How to calculate the number of atoms in a sample. Equivalently, first find the number of atoms in $1$ cubic.
Solved Find the number of atoms per square centimeter in
How Many Atoms Are In A Centimeter How to calculate the number of atoms in a sample. Equivalently, first find the number of atoms in $1$ cubic. How to calculate the number of atoms in a sample. Today, they agree that atoms have a. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. To calculate the number of atoms in a. Scientists’ ideas about atoms have changed over time. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Divide this by the mass of an atom to find, approximately, the number of atoms. Converting both units to meters and using scientific.
From abcinfolinks.blogspot.com
abc blog Discovery of an ATOM. How Many Atoms Are In A Centimeter The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Scientists’ ideas about atoms have changed over time. Converting both units to meters and using scientific. How to calculate the number of atoms in a sample. Chemists have chosen to count atoms and molecules using. How Many Atoms Are In A Centimeter.
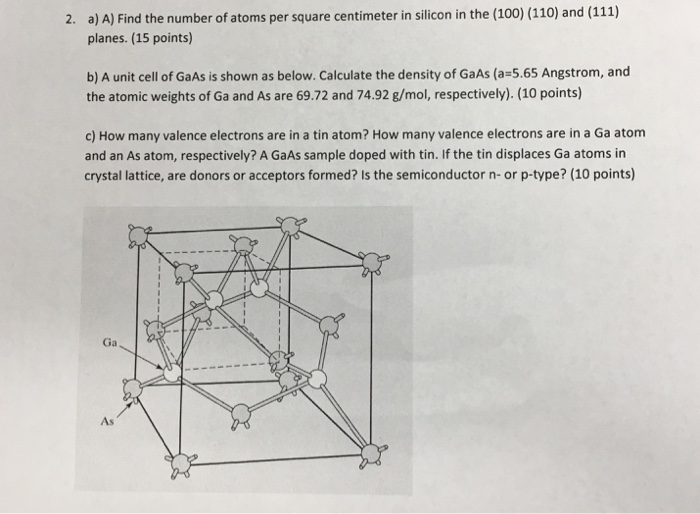
From lessonlibrarysnotter.z13.web.core.windows.net
Formula For Calculating Number Of Atoms How Many Atoms Are In A Centimeter Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. To calculate the number of atoms in a. Divide this by the mass of an atom to find, approximately, the number of atoms. Chemists have chosen to count atoms and molecules using a unit called the. How Many Atoms Are In A Centimeter.
From www.nagwa.com
Question Video Calculating the Concentration of Free Electrons for a How Many Atoms Are In A Centimeter Converting both units to meters and using scientific. Divide this by the mass of an atom to find, approximately, the number of atoms. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Scientists’ ideas about atoms have changed over time. Equivalently, first find the. How Many Atoms Are In A Centimeter.
From www.bartleby.com
Answered The density of solid Fe is 7.87 g/cm.… bartleby How Many Atoms Are In A Centimeter Today, they agree that atoms have a. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Converting both units to. How Many Atoms Are In A Centimeter.
From www.youtube.com
grams to atoms calculations YouTube How Many Atoms Are In A Centimeter Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. To calculate the number of atoms in a. Equivalently, first find the number. How Many Atoms Are In A Centimeter.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Moles Using Grams How Many Atoms Are In A Centimeter The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Converting both units to meters and using scientific. Scientists’ ideas about atoms have changed over time. Divide this by the mass of an atom to find, approximately, the number of atoms. Equivalently, first find the. How Many Atoms Are In A Centimeter.
From www.numerade.com
SOLVED The density of solid fe is 7.87 g/cm3. how many atoms are How Many Atoms Are In A Centimeter To calculate the number of atoms in a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Divide this by the mass of an atom to find, approximately, the number of atoms. How to calculate the number of atoms in a sample. Equivalently, first find the. How Many Atoms Are In A Centimeter.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID472642 How Many Atoms Are In A Centimeter Today, they agree that atoms have a. Converting both units to meters and using scientific. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Divide this by the mass of an atom to find, approximately, the number of atoms. Equivalently, first find the number of. How Many Atoms Are In A Centimeter.
From www.chegg.com
Solved Find the number of atoms per square centimeter in How Many Atoms Are In A Centimeter Divide this by the mass of an atom to find, approximately, the number of atoms. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Equivalently, first find the number of atoms in $1$ cubic. Chemists have chosen to count atoms and molecules using a unit. How Many Atoms Are In A Centimeter.
From taradesnhrandolph.blogspot.com
How Many Atoms Are in 2.5 Moles of Gold How Many Atoms Are In A Centimeter Converting both units to meters and using scientific. Today, they agree that atoms have a. Equivalently, first find the number of atoms in $1$ cubic. Scientists’ ideas about atoms have changed over time. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. How to. How Many Atoms Are In A Centimeter.
From www.youtube.com
How many atoms of H are there in 28g of water? YouTube How Many Atoms Are In A Centimeter The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Today, they agree that atoms have a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Divide this by the mass. How Many Atoms Are In A Centimeter.
From www.numerade.com
SOLVED A Si sample contains 1016 cm3 In acceptor atoms and a certain How Many Atoms Are In A Centimeter How to calculate the number of atoms in a sample. Scientists’ ideas about atoms have changed over time. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Equivalently, first find the number of atoms in $1$ cubic. Divide this by the mass of an atom to. How Many Atoms Are In A Centimeter.
From www.chegg.com
Solved Consider a bodycentered cubic unit cell as shown How Many Atoms Are In A Centimeter Scientists’ ideas about atoms have changed over time. Today, they agree that atoms have a. Equivalently, first find the number of atoms in $1$ cubic. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. To calculate the number of atoms in a. Converting both. How Many Atoms Are In A Centimeter.
From physics-madeeasy.blogspot.com
17. (II) A typical atom has a diameter of about 1.0 X 10^10m. (a) What How Many Atoms Are In A Centimeter Converting both units to meters and using scientific. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Equivalently, first find. How Many Atoms Are In A Centimeter.
From www.pinterest.com
Scale of Things from 100 to 1 centimeter. In case you How Many Atoms Are In A Centimeter Equivalently, first find the number of atoms in $1$ cubic. Converting both units to meters and using scientific. How to calculate the number of atoms in a sample. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Scientists’ ideas about atoms have changed over time.. How Many Atoms Are In A Centimeter.
From lessonlibrarysnotter.z13.web.core.windows.net
How To Calculate Number Of Atoms How Many Atoms Are In A Centimeter Divide this by the mass of an atom to find, approximately, the number of atoms. Converting both units to meters and using scientific. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Scientists’ ideas about atoms have changed over time. Today, they agree that atoms have. How Many Atoms Are In A Centimeter.
From www.youtube.com
How To Calculate The Number of Atoms Chemistry YouTube How Many Atoms Are In A Centimeter To calculate the number of atoms in a. Today, they agree that atoms have a. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Divide this by the mass of an atom to find, approximately, the number of atoms. Suppose you compare an atom. How Many Atoms Are In A Centimeter.
From www.youtube.com
Practice Problem Copper Atom YouTube How Many Atoms Are In A Centimeter Converting both units to meters and using scientific. Today, they agree that atoms have a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of. How Many Atoms Are In A Centimeter.
From www.aiophotoz.com
How To Find Number Of Atoms Images and Photos finder How Many Atoms Are In A Centimeter To calculate the number of atoms in a. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Equivalently, first find the. How Many Atoms Are In A Centimeter.
From chem.libretexts.org
12.2 The Arrangement of Atoms in Crystalline Solids Chemistry LibreTexts How Many Atoms Are In A Centimeter Divide this by the mass of an atom to find, approximately, the number of atoms. Scientists’ ideas about atoms have changed over time. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. To calculate the number of atoms in a. Converting both units to meters. How Many Atoms Are In A Centimeter.
From www.sciencephoto.com
Gold, atomic structure Stock Image C018/3760 Science Photo Library How Many Atoms Are In A Centimeter How to calculate the number of atoms in a sample. Today, they agree that atoms have a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Scientists’ ideas about atoms have changed over time. Converting both units to meters and using scientific. Suppose you compare an. How Many Atoms Are In A Centimeter.
From www.pedalaman.com
3 Ways To Convert Inches To Centimeters Pedalaman How Many Atoms Are In A Centimeter Scientists’ ideas about atoms have changed over time. Equivalently, first find the number of atoms in $1$ cubic. How to calculate the number of atoms in a sample. Divide this by the mass of an atom to find, approximately, the number of atoms. Converting both units to meters and using scientific. Chemists have chosen to count atoms and molecules using. How Many Atoms Are In A Centimeter.
From www.numerade.com
SOLVED The radius of an atom of gold (Au) is about 1.35 Ã…. (a How Many Atoms Are In A Centimeter To calculate the number of atoms in a. Today, they agree that atoms have a. Converting both units to meters and using scientific. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. The size of atoms can be estimated with the use of avogadro's number along. How Many Atoms Are In A Centimeter.
From www.sliderbase.com
The Atom Presentation Chemistry How Many Atoms Are In A Centimeter Scientists’ ideas about atoms have changed over time. Equivalently, first find the number of atoms in $1$ cubic. Divide this by the mass of an atom to find, approximately, the number of atoms. To calculate the number of atoms in a. How to calculate the number of atoms in a sample. Suppose you compare an atom that has a diameter. How Many Atoms Are In A Centimeter.
From www.semanticscholar.org
Figure 2 from SingleSource Multiaxis ColdAtom Interferometer in a How Many Atoms Are In A Centimeter Equivalently, first find the number of atoms in $1$ cubic. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Scientists’ ideas about atoms have changed over time. Divide this by the mass of an atom to find, approximately, the number of atoms. Converting both units. How Many Atoms Are In A Centimeter.
From slideplayer.com
Lesson Overview 2.1 The Nature of Matter. ppt download How Many Atoms Are In A Centimeter Equivalently, first find the number of atoms in $1$ cubic. How to calculate the number of atoms in a sample. To calculate the number of atoms in a. Today, they agree that atoms have a. Scientists’ ideas about atoms have changed over time. Converting both units to meters and using scientific. Suppose you compare an atom that has a diameter. How Many Atoms Are In A Centimeter.
From theeducationinfo.com
Grams to atoms calculator step by step guide How Many Atoms Are In A Centimeter Divide this by the mass of an atom to find, approximately, the number of atoms. Scientists’ ideas about atoms have changed over time. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Equivalently, first find the number of atoms in $1$ cubic. Suppose you. How Many Atoms Are In A Centimeter.
From studytrainatora9.z21.web.core.windows.net
Identify The Substances That Are Molecules How Many Atoms Are In A Centimeter The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. Converting both units to meters and using scientific. How to calculate. How Many Atoms Are In A Centimeter.
From slideplayer.com
Lesson Overview 2.1 The Nature of Matter. ppt download How Many Atoms Are In A Centimeter Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a diameter of 1 cm. To calculate the number of atoms in a. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Today, they agree that atoms. How Many Atoms Are In A Centimeter.
From www.researchgate.net
Scale of science from millimeter (mm) to nanometer (nm) Download How Many Atoms Are In A Centimeter Equivalently, first find the number of atoms in $1$ cubic. Today, they agree that atoms have a. To calculate the number of atoms in a. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Converting both units to meters and using scientific. Suppose you. How Many Atoms Are In A Centimeter.
From www.youtube.com
How Big are Atoms (Size Comparison) Atoms and Molecules 5 in How Many Atoms Are In A Centimeter To calculate the number of atoms in a. Today, they agree that atoms have a. Divide this by the mass of an atom to find, approximately, the number of atoms. Scientists’ ideas about atoms have changed over time. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of. How Many Atoms Are In A Centimeter.
From www.youtube.com
Counting the Number of Atoms in a Molecule YouTube How Many Atoms Are In A Centimeter Equivalently, first find the number of atoms in $1$ cubic. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. Converting both units to meters and using scientific. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has. How Many Atoms Are In A Centimeter.
From www.numerade.com
SOLVED 1. How many silicon atoms in a cubic centimeter? (the answer is How Many Atoms Are In A Centimeter The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid material. Converting both units to meters and using scientific. Scientists’ ideas about atoms have changed over time. To calculate the number of atoms in a. Today, they agree that atoms have a. How to calculate the. How Many Atoms Are In A Centimeter.
From www.youtube.com
Moles To Atoms Conversion Chemistry YouTube How Many Atoms Are In A Centimeter Divide this by the mass of an atom to find, approximately, the number of atoms. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the latin moles, meaning “pile” or “heap.”. The size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of. How Many Atoms Are In A Centimeter.
From www.youtube.com
Finding radius and volume of atom from density and molar mass YouTube How Many Atoms Are In A Centimeter How to calculate the number of atoms in a sample. Today, they agree that atoms have a. Divide this by the mass of an atom to find, approximately, the number of atoms. Converting both units to meters and using scientific. Suppose you compare an atom that has a diameter of 0.1 nm with a size aaa battery that has a. How Many Atoms Are In A Centimeter.