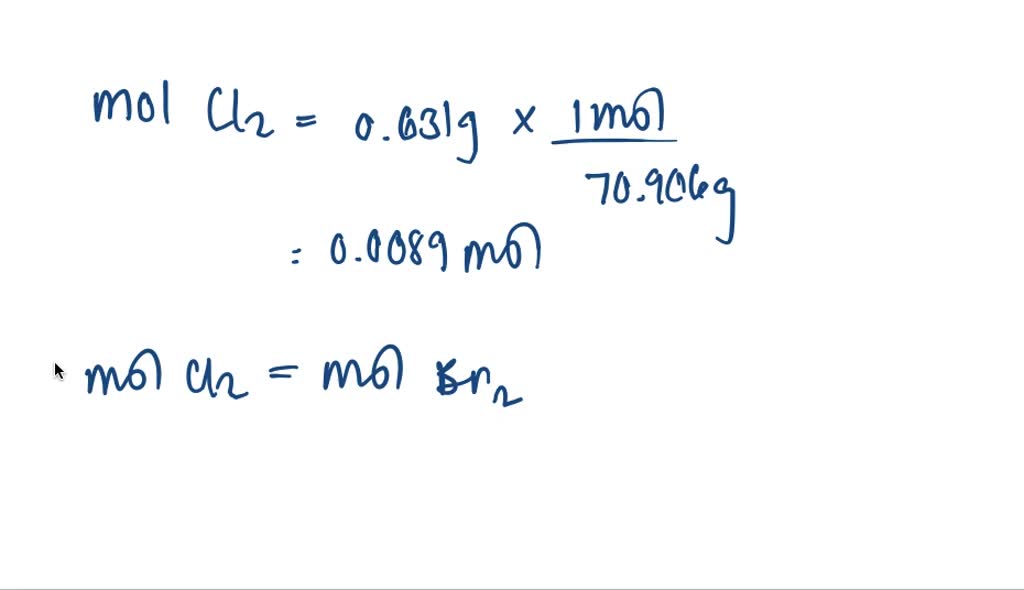Bromine + Potassium Chloride Equation . The slideshow shows what happens when solutions of chlorine, bromine. The equation for the reaction between bromine and potassium chloride is: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Chlorine reacts with potassium bromide to form potassium chloride and bromine. In this reaction chlorine forms chloride ions: The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Chlorine + potassium bromide → bromine + potassium chloride:
from www.numerade.com
Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Chlorine reacts with potassium bromide to form potassium chloride and bromine. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. The slideshow shows what happens when solutions of chlorine, bromine. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The equation for the reaction between bromine and potassium chloride is: In this reaction chlorine forms chloride ions: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous.
SOLVEDGaseous chlorine will displace bromide ion from an aqueous
Bromine + Potassium Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride: The equation for the reaction between bromine and potassium chloride is: Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when solutions of chlorine, bromine. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. In this reaction chlorine forms chloride ions: Chlorine reacts with potassium bromide to form potassium chloride and bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts.
From www.slideshare.net
Chemical Reactions Bromine + Potassium Chloride Equation Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The equation for the reaction between bromine and potassium chloride is: In this reaction chlorine forms chloride ions: The chlorine has displaced the bromine from solution as it is more reactive which can be summarised. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Chlorine can replace bromine in bromide compounds, forming Bromine + Potassium Chloride Equation The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2. Bromine + Potassium Chloride Equation.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Bromine + Potassium Chloride Equation Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where. Bromine + Potassium Chloride Equation.
From internetfriends.web.fc2.com
bromine gas chemical formula Bromine + Potassium Chloride Equation The slideshow shows what happens when solutions of chlorine, bromine. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The equation for the reaction. Bromine + Potassium Chloride Equation.
From ceukyrig.blob.core.windows.net
Aqueous Zinc Bromide Equation at Heather Spiers blog Bromine + Potassium Chloride Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid. Bromine + Potassium Chloride Equation.
From cesfmvkc.blob.core.windows.net
Liquid Bromine Is Added To A Solution Of Potassium Iodide at Steven Bromine + Potassium Chloride Equation The equation for the reaction between bromine and potassium chloride is: The slideshow shows what happens when solutions of chlorine, bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and. Bromine + Potassium Chloride Equation.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction of Bromine + Potassium Chloride Equation Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction. Bromine + Potassium Chloride Equation.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine + Potassium Chloride Equation Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The equation for the reaction between bromine and potassium chloride is: The slideshow shows what happens when solutions of chlorine, bromine. In this reaction chlorine forms chloride ions: Bromine only displaces iodine from potassium iodide. Bromine + Potassium Chloride Equation.
From www.numerade.com
worksheet 4 translating word equations into formula equations and vice Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Chlorine reacts with potassium bromide to form potassium chloride and bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution. Bromine + Potassium Chloride Equation.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU1P1220 Bromine + Potassium Chloride Equation Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. The equation for the reaction between bromine and potassium chloride is: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The. Bromine + Potassium Chloride Equation.
From xaydungso.vn
Khám Phá Phản Ứng Hóa Học CL2 + KBR Tính Chất, Ứng Dụng và An Toàn Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: The slideshow shows what happens when solutions of chlorine, bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles. Bromine + Potassium Chloride Equation.
From loeztynlp.blob.core.windows.net
Can You Mix Chlorine And Bromine In A Pool at Alex Finck blog Bromine + Potassium Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Chlorine reacts with potassium bromide to form potassium chloride and bromine. In this reaction chlorine forms chloride ions: Cl 2 (aq) + 2ki (aq) → 2kcl. Bromine + Potassium Chloride Equation.
From brainly.com
Chlorine gas reacts with aqueous sodium bromide to produce aqueous Bromine + Potassium Chloride Equation Chlorine reacts with potassium bromide to form potassium chloride and bromine. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The slideshow shows what happens when solutions of chlorine, bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace. Bromine + Potassium Chloride Equation.
From loetrsxww.blob.core.windows.net
Hydrolysis Of Iron(Iii) Chloride Equation at Lamphere blog Bromine + Potassium Chloride Equation Chlorine reacts with potassium bromide to form potassium chloride and bromine. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine + potassium bromide → bromine +. Bromine + Potassium Chloride Equation.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine + potassium bromide → bromine + potassium chloride: Br. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine + Potassium Chloride Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The slideshow shows what happens when solutions of chlorine, bromine. Chlorine + potassium bromide → bromine + potassium. Bromine + Potassium Chloride Equation.
From studylib.net
Displacement reactions Reactions of chlorine Bromine + Potassium Chloride Equation Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The slideshow shows what. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Write equations for the halfreactions that occur in the Bromine + Potassium Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Kbr + cl = kcl. Bromine + Potassium Chloride Equation.
From www.thesciencehive.co.uk
Group7halogensanswers — the science sauce Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) +. Bromine + Potassium Chloride Equation.
From www.youtube.com
Equation for KBr + H2O (Potassium bromide + Water) YouTube Bromine + Potassium Chloride Equation Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. The slideshow shows what happens when solutions of chlorine, bromine. Bromine only. Bromine + Potassium Chloride Equation.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous Bromine + Potassium Chloride Equation The slideshow shows what happens when solutions of chlorine, bromine. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Cl 2 (aq) + 2ki. Bromine + Potassium Chloride Equation.
From www.nagwa.com
Question Video Identifying the Chemical Equation That Describes the Bromine + Potassium Chloride Equation In this reaction chlorine forms chloride ions: The equation for the reaction between bromine and potassium chloride is: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Comparing the chemical formula of compounds formed with Bromine + Potassium Chloride Equation The equation for the reaction between bromine and potassium chloride is: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. The slideshow shows what happens when solutions of. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine + Potassium Chloride Equation Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two. Bromine + Potassium Chloride Equation.
From gioxjnupq.blob.core.windows.net
Potassium Bromide Electronegativity Difference at Maria Tosh blog Bromine + Potassium Chloride Equation Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Chlorine reacts with potassium bromide to form potassium chloride and bromine. In this reaction. Bromine + Potassium Chloride Equation.
From www.bartleby.com
Answered Complete the table below by deciding… bartleby Bromine + Potassium Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. In this reaction chlorine forms chloride ions: The equation for the reaction between bromine and potassium chloride is: Chlorine reacts with potassium bromide to form potassium. Bromine + Potassium Chloride Equation.
From www.bharatagritech.com
What Is A Chemical Equation For The Reaction Between, 52 OFF Bromine + Potassium Chloride Equation The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. In this reaction chlorine forms chloride ions: Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Chlorine reacts with potassium bromide to form potassium chloride. Bromine + Potassium Chloride Equation.
From byjus.com
Write the chemical formulae of the following a Magnesium chloride Bromine + Potassium Chloride Equation Chlorine + potassium bromide → bromine + potassium chloride: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The equation for the reaction between bromine and potassium chloride is: The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in. Bromine + Potassium Chloride Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine + Potassium Chloride Equation Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Chlorine + potassium bromide → bromine + potassium chloride: Br + kcl = kbr + cl2. Bromine + Potassium Chloride Equation.
From brainly.in
Chlorine reacts with potassium bromide. complete the word equation Bromine + Potassium Chloride Equation Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. The equation for the reaction between bromine and potassium chloride is: Chlorine. Bromine + Potassium Chloride Equation.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Bromine + Potassium Chloride Equation The slideshow shows what happens when solutions of chlorine, bromine. The equation for the reaction between bromine and potassium chloride is: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Chlorine + potassium bromide → bromine + potassium chloride: Chlorine reacts with potassium bromide to form potassium chloride and. Bromine + Potassium Chloride Equation.
From giomwwywg.blob.core.windows.net
Potassium Bromide Ionic at Bruce Pruitt blog Bromine + Potassium Chloride Equation Kbr + cl = kcl + br2 is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and two. The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. The slideshow shows what happens when solutions of chlorine, bromine. Chlorine reacts with potassium bromide to. Bromine + Potassium Chloride Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine + Potassium Chloride Equation The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic. Br + kcl = kbr + cl2 is a single displacement (substitution) reaction where two moles of liquid bromine [br] and two moles of aqueous. Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace. Bromine + Potassium Chloride Equation.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Bromine + Potassium Chloride Equation Chlorine reacts with potassium bromide to form potassium chloride and bromine. In this reaction chlorine forms chloride ions: Bromine only displaces iodine from potassium iodide and the least reactive iodine cannot displace chlorine or bromine from their salts. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms.. Bromine + Potassium Chloride Equation.