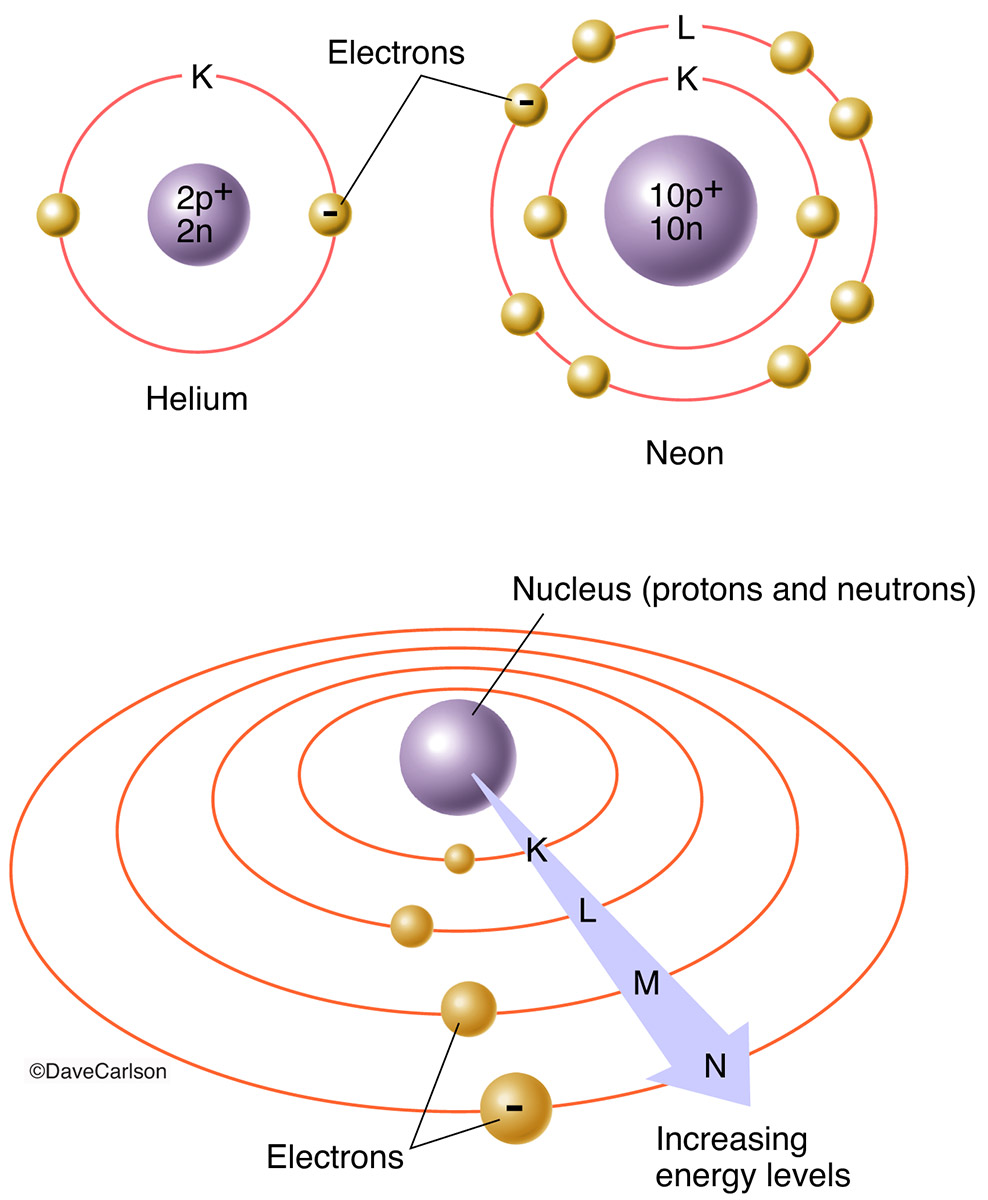
Energy Levels Electron . Shown here is the first balmer transition, in which an electron jumps from. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. As you go farther from the nucleus, electrons at higher. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Electrons in energy level i (also called energy level k) have the least amount of energy. According to bohr's theory, electrons of. However, electrons will never be found in between.
from
Shown here is the first balmer transition, in which an electron jumps from. As you go farther from the nucleus, electrons at higher. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. However, electrons will never be found in between. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Electrons in energy level i (also called energy level k) have the least amount of energy. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
Energy Levels Electron Shown here is the first balmer transition, in which an electron jumps from. As you go farther from the nucleus, electrons at higher. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. According to bohr's theory, electrons of. Electrons in energy level i (also called energy level k) have the least amount of energy. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. However, electrons will never be found in between. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Shown here is the first balmer transition, in which an electron jumps from.
From
Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy. Energy Levels Electron.
From
Energy Levels Electron Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. As you go farther from the nucleus, electrons at higher. Shown here is the first balmer transition, in which an electron jumps from. However, electrons will never be found in between. In this section. Energy Levels Electron.
From
Energy Levels Electron Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electrons in energy level i (also called energy level k) have the least amount of energy. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these. Energy Levels Electron.
From
Energy Levels Electron In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. As you go farther from the nucleus, electrons at higher. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can.. Energy Levels Electron.
From
Energy Levels Electron In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to bohr's theory, electrons of. Electron shells are also. Energy Levels Electron.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Energy Levels Electron Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. When an electron jumps from a higher energy level to a lower one, it emits. Energy Levels Electron.
From guidedehartsicklewort.z21.web.core.windows.net
Orbital Diagram Of Elements Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. However, electrons will never be found in between. Shown here is the first balmer transition, in which an electron jumps from. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. Energy Levels Electron.
From
Energy Levels Electron When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. In this explainer, we will learn how to describe and identify energy levels in. Energy Levels Electron.
From
Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. However, electrons will never be found in between. As you go farther from the nucleus, electrons at higher. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Electron. Energy Levels Electron.
From
Energy Levels Electron Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. However, electrons will never be found in between. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. According to bohr's theory, electrons of. Electrons in energy level i (also. Energy Levels Electron.
From
Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. However, electrons will never be found in between. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to. Energy Levels Electron.
From scientifictutor.org
Chem Complete Electron Configurations Scientific Tutor Energy Levels Electron In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to bohr's theory, electrons of. Shown here is the first balmer transition, in which an electron jumps from. Energy is emitted from the atom when the electron jumps from one orbit to another closer. Energy Levels Electron.
From
Energy Levels Electron Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Shown here is the first balmer transition, in which an electron jumps from. Electron. Energy Levels Electron.
From
Energy Levels Electron Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. According to bohr's theory, electrons of. Electrons in energy level i (also called energy level k) have the least amount. Energy Levels Electron.
From www.savemyexams.co.uk
Electron Configuration (1.1.5) AQA A Level Chemistry Revision Notes Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. In this section we. Energy Levels Electron.
From study.com
Valence Electrons Definition, Role & Examples Video & Lesson Energy Levels Electron Electrons in energy level i (also called energy level k) have the least amount of energy. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Electrons can either jump. Energy Levels Electron.
From
Energy Levels Electron In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Electron shells are also called electron energy levels. Energy Levels Electron.
From
Energy Levels Electron Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. Shown here is the first balmer transition, in which an electron jumps from. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell.. Energy Levels Electron.
From
Energy Levels Electron When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can. Electrons can either jump to a higher energy. Energy Levels Electron.
From
Energy Levels Electron Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. As you go farther from the nucleus, electrons at. Energy Levels Electron.
From www.naturphilosophie.co.uk
At the Heart of the Hydrogen Atom... NaturPhilosophie Energy Levels Electron In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. However, electrons will never be found in between. Energy is emitted from the atom. Energy Levels Electron.
From
Energy Levels Electron According to bohr's theory, electrons of. Electrons in energy level i (also called energy level k) have the least amount of energy. Shown here is the first balmer transition, in which an electron jumps from. In this explainer, we will learn how to describe and identify energy levels in atoms and determine the number of electrons each energy level can.. Energy Levels Electron.
From
Energy Levels Electron Shown here is the first balmer transition, in which an electron jumps from. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. As. Energy Levels Electron.
From
Energy Levels Electron Shown here is the first balmer transition, in which an electron jumps from. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to. Energy Levels Electron.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art Energy Levels Electron According to bohr's theory, electrons of. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. However, electrons will never be found in between. As you go farther from the nucleus, electrons at higher. Shown here is the first balmer transition, in which. Energy Levels Electron.
From wiringfixpicketing.z21.web.core.windows.net
Energy Diagram For Hydrogen Atom Energy Levels Electron Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. As you go farther from the nucleus,. Energy Levels Electron.
From
Energy Levels Electron When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. According to bohr's theory, electrons of. Electron shells are also called electron energy levels. Energy Levels Electron.
From
Energy Levels Electron When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Electrons in energy level i (also called energy level k) have the least amount of energy. In this explainer, we will learn how to describe and identify energy levels in atoms and determine. Energy Levels Electron.
From
Energy Levels Electron However, electrons will never be found in between. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. As you go farther from the nucleus, electrons at higher. Shown here is the first balmer transition, in which an electron jumps from. Electron shells are also. Energy Levels Electron.
From ar.inspiredpencil.com
Electron Energy Levels Energy Levels Electron In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electrons in energy level i (also called energy level k) have the least amount of energy. Shown here is the first balmer transition, in which an electron jumps from. Electron shells are also called electron. Energy Levels Electron.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons Energy Levels Electron According to bohr's theory, electrons of. However, electrons will never be found in between. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it. Energy Levels Electron.
From
Energy Levels Electron In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. When an electron jumps from a higher energy level. Energy Levels Electron.
From
Energy Levels Electron Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. According to bohr's theory, electrons of. Shown here is the first balmer transition, in which an electron jumps from. However, electrons will never be found in between. In this explainer, we will learn how to describe and identify energy levels in. Energy Levels Electron.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID5479879 Energy Levels Electron Shown here is the first balmer transition, in which an electron jumps from. When an electron jumps from a higher energy level to a lower one, it emits a photon with energy equal to the difference between these two levels. Electrons in energy level i (also called energy level k) have the least amount of energy. In this explainer, we. Energy Levels Electron.
From www.chegg.com
An electron is bound in a square well with a depth Energy Levels Electron Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electron shells are also called electron energy levels because the energy of the electrons within them changes from shell to shell. Electrons in energy level i (also called energy level k) have the least amount of energy. However, electrons will never. Energy Levels Electron.