Titration How To Do It . Find examples of titration experiments and tips. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Place the conical flask with solution a. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. When you have filled the burette and pipette, carry out the titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn how to perform and interpret titration. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until.
from mmerevise.co.uk
Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Learn how to perform and interpret titration. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. When you have filled the burette and pipette, carry out the titration. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Place the conical flask with solution a. Find examples of titration experiments and tips. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs.
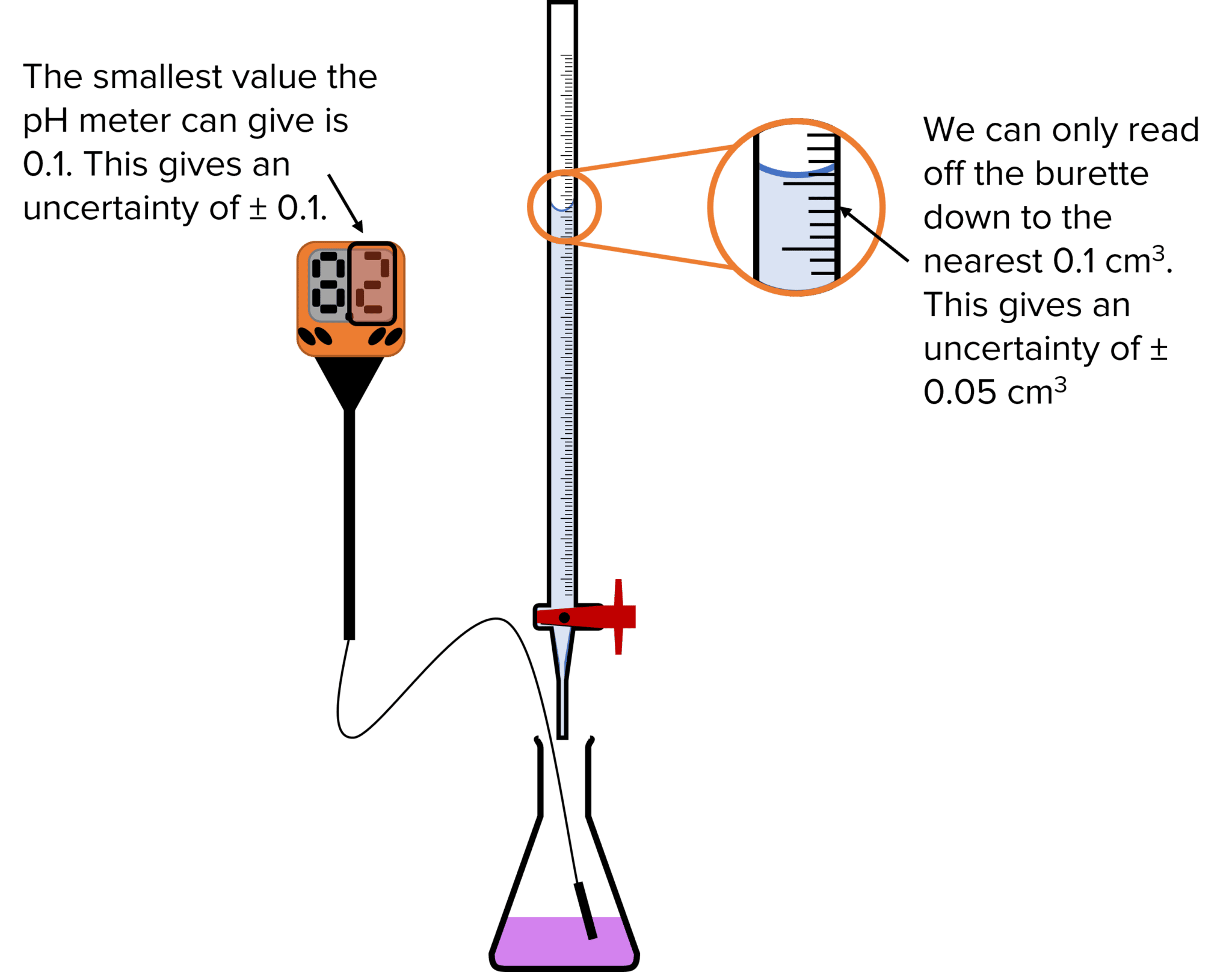
Titrations and Uncertainties MME
Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Place the conical flask with solution a. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. When you have filled the burette and pipette, carry out the titration. Find examples of titration experiments and tips. Learn how to perform and interpret titration.
From ar.inspiredpencil.com
Titration Diagram Titration How To Do It When you have filled the burette and pipette, carry out the titration. Find examples of titration experiments and tips. Place the conical flask with solution a. Learn how to perform and interpret titration. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. A titration is a volumetric technique to determine the. Titration How To Do It.
From fyodcnnui.blob.core.windows.net
Titration A Level Chemistry Ocr at David Lackey blog Titration How To Do It When you have filled the burette and pipette, carry out the titration. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Titration is a method to precisely determine the amount of a substance (analyte) by reacting. Titration How To Do It.
From exypvoihf.blob.core.windows.net
Discussion For Titration at Jacqueline Demaio blog Titration How To Do It A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Place the conical flask with solution a. Learn the principle, reagents and apparatus used, and how to carry out a. Titration How To Do It.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn the principle, reagents and apparatus used, and how to carry out. Titration How To Do It.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration How To Do It Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Find examples of titration experiments and tips. Place the conical flask with solution a. Learn how to perform and interpret titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Titration is. Titration How To Do It.
From www.chegg.com
Solved Question 29is the titration of iodine produced by a Titration How To Do It Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a method to precisely determine the amount. Titration How To Do It.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration How To Do It Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. A titration is a. Titration How To Do It.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration How To Do It When you have filled the burette and pipette, carry out the titration. Learn how to perform and interpret titration. Place the conical flask with solution a. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume. Titration How To Do It.
From exohkacwb.blob.core.windows.net
End Point Of Titration Moles at Melvin Jolly blog Titration How To Do It Find examples of titration experiments and tips. Place the conical flask with solution a. Learn how to perform and interpret titration. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. When you have filled. Titration How To Do It.
From exyyylzwb.blob.core.windows.net
Titration In Pharmaceutical Industry at Jamel Edwards blog Titration How To Do It Find examples of titration experiments and tips. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. When you have filled the burette and pipette, carry out. Titration How To Do It.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration How To Do It Learn how to perform and interpret titration. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Place the conical flask with solution a. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Titration is a method to precisely determine the amount of a. Titration How To Do It.
From theedge.com.hk
Chemistry How To Titration The Edge Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Find examples of titration experiments and tips. Place the conical flask with solution a. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard. Titration How To Do It.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration How To Do It Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Find. Titration How To Do It.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn what titration is, how it works, and how to use indicators. Titration How To Do It.
From www.reddit.com
First Time Doing Titrations How Did I Do? r/chemistry Titration How To Do It Find examples of titration experiments and tips. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. When you have filled the burette and pipette, carry out the titration. Learn. Titration How To Do It.
From exohtntjr.blob.core.windows.net
How To Calculate Titration Value at Eric Whitlow blog Titration How To Do It Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. When you have filled the burette and pipette, carry out the titration. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a laboratory technique to measure molar concentration of. Titration How To Do It.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration How To Do It Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Place the conical flask with solution a. When you. Titration How To Do It.
From solvely.ai
The titration curve of HCl and NaOH is shown below Solvely Titration How To Do It Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Place the conical flask with solution a. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of. Titration How To Do It.
From www.makpa.com
predchodca sklo hladoval reverse titration calculation odvolanie papraď Titration How To Do It A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. When you have filled the burette and pipette, carry out the titration. Find examples of titration experiments and tips. A. Titration How To Do It.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Place the conical flask with solution a. When you have filled the burette and pipette, carry out the titration. A titration is a laboratory technique to measure molar concentration of an unknown solution. Titration How To Do It.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration How To Do It Find examples of titration experiments and tips. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Place the conical flask with solution a. Titration is a method to precisely determine the amount of a substance. Titration How To Do It.
From exoqvrery.blob.core.windows.net
Introduction To Acid Base Titration Lab at Christopher Beatty blog Titration How To Do It Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Learn how to perform and interpret titration. Place the conical flask with solution a. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn what titration is, how it works, and how to use. Titration How To Do It.
From www.chegg.com
Calculate the pH during the titration of 50.00mL of Titration How To Do It A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Place the conical flask with solution a. Learn what titration is, how it works, and how to use indicators like. Titration How To Do It.
From www.science-revision.co.uk
Titrations Titration How To Do It Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Learn how to perform and interpret titration. When you have filled. Titration How To Do It.
From mavink.com
Titration Picture Titration How To Do It Learn how to perform and interpret titration. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Find examples of titration experiments and tips. Learn. Titration How To Do It.
From www.thecannabiscommunity.org
What Is Titration? Benefits, Tips and How To Do It Right Titration How To Do It Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. A titration is a volumetric technique to determine the concentration of a solution by adding a known. Titration How To Do It.
From www.compoundchem.com
Compound Interest Chemistry Techniques Titration Titration How To Do It When you have filled the burette and pipette, carry out the titration. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn how to perform and interpret titration. A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Learn the. Titration How To Do It.
From ar.inspiredpencil.com
Titration Phenolphthalein Titration How To Do It Place the conical flask with solution a. Find examples of titration experiments and tips. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Learn how to perform and interpret titration. Titration is a method to precisely determine the amount of a substance. Titration How To Do It.
From www.scribd.com
Titration PDF Titration Chemistry Titration How To Do It Find examples of titration experiments and tips. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. A titration is a volumetric technique to determine the concentration of a solution by. Titration How To Do It.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Titration How To Do It Place the conical flask with solution a. Find examples of titration experiments and tips. A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. A titration is. Titration How To Do It.
From fyongsdbl.blob.core.windows.net
How To Do Acid Base Titration at Marilyn Hyde blog Titration How To Do It Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn what titration is, how it works, and how to use indicators like starch and phenolphthalein. Place the conical flask with solution a. A titration is a volumetric technique to determine the concentration of a solution by adding a. Titration How To Do It.
From mungfali.com
Acid Base Titration Calculation Titration How To Do It A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Find examples of titration experiments and tips. A titration is a volumetric technique to determine the concentration of a solution by. Titration How To Do It.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog Titration How To Do It A titration is a volumetric technique to determine the concentration of a solution by adding a known volume of another solution until the equivalence point is reached. Place the conical flask with solution a. When you have filled the burette and pipette, carry out the titration. Titration is a technique to determine the concentration of an unknown solution by adding. Titration How To Do It.
From www.youtube.com
How to do back titrations also known as indirect titrations. YouTube Titration How To Do It When you have filled the burette and pipette, carry out the titration. Learn how to perform and interpret titration. Titration is a method to precisely determine the amount of a substance (analyte) by reacting it with a standard solution (titrant) until. Learn the principle, reagents and apparatus used, and how to carry out a titration step by step. Find examples. Titration How To Do It.
From www.youtube.com
Back Titration Example YouTube Titration How To Do It A titration is a laboratory technique to measure molar concentration of an unknown solution using a standard solution. Place the conical flask with solution a. When you have filled the burette and pipette, carry out the titration. Find examples of titration experiments and tips. Learn how to perform and interpret titration. Learn what titration is, how it works, and how. Titration How To Do It.