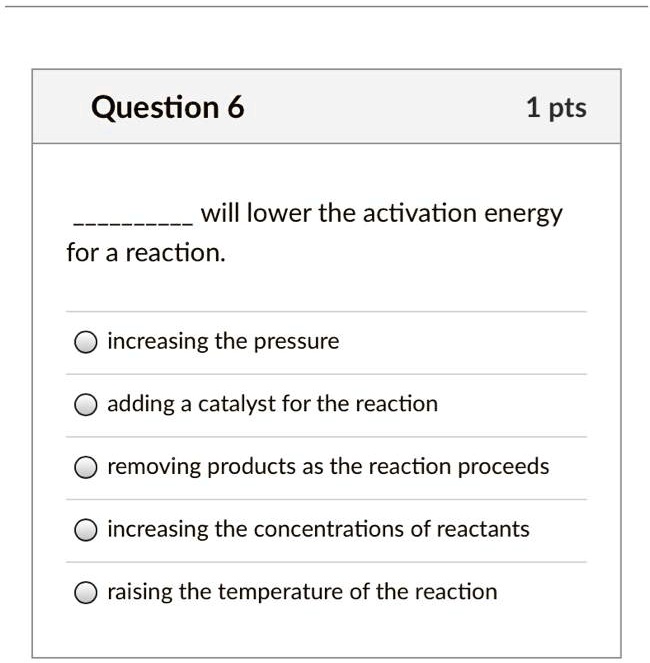
Will Lower The Activation Energy For A Reaction . Orientating reactants correctly in space for. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. How to amazons lower the activation energy of chemical reaction? The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. Select all that apply a. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. If a reaction has a low activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The problem is that the activation energy is not derived the way you think. It is obtained by measuring a reaction rate constant at different temperatures.
from www.numerade.com
Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. The problem is that the activation energy is not derived the way you think. Orientating reactants correctly in space for. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If a reaction has a low activation energy. Select all that apply a. How to amazons lower the activation energy of chemical reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. It is obtained by measuring a reaction rate constant at different temperatures.
SOLVED Question 6 1 pts will lower the activation energy for a
Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. Select all that apply a. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. How to amazons lower the activation energy of chemical reaction? If a reaction has a low activation energy. It is obtained by measuring a reaction rate constant at different temperatures. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. The problem is that the activation energy is not derived the way you think. Orientating reactants correctly in space for.
From www.nagwa.com
Question Video Identifying the Activation Energy of an Enzyme Will Lower The Activation Energy For A Reaction The problem is that the activation energy is not derived the way you think. How to amazons lower the activation energy of chemical reaction? In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so. Will Lower The Activation Energy For A Reaction.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Will Lower The Activation Energy For A Reaction It is obtained by measuring a reaction rate constant at different temperatures. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. Will Lower The Activation Energy For A Reaction.
From byjus.com
The forward and backward activation energy of exothermic reaction A→ B Will Lower The Activation Energy For A Reaction Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. Select all that apply a. In chemistry, activation energy is the minimum. Will Lower The Activation Energy For A Reaction.
From byjus.com
How rate of reaction depends on activation energy Will Lower The Activation Energy For A Reaction Orientating reactants correctly in space for. It is obtained by measuring a reaction rate constant at different temperatures. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If a reaction has a low activation energy. The problem is that the. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved The figure illustrates the energy states associated Will Lower The Activation Energy For A Reaction Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. Select all that apply a. The problem is that. Will Lower The Activation Energy For A Reaction.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Will Lower The Activation Energy For A Reaction If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. How to amazons lower the activation energy of chemical reaction? Select all that apply a. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. Will Lower The Activation Energy For A Reaction.
From www.varsitytutors.com
Reaction Coordinate Diagrams College Chemistry Will Lower The Activation Energy For A Reaction Orientating reactants correctly in space for. Select all that apply a. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. It is obtained by measuring a reaction rate constant at different temperatures. If the activation energy is high for a. Will Lower The Activation Energy For A Reaction.
From www.numerade.com
SOLVED Question 6 1 pts will lower the activation energy for a Will Lower The Activation Energy For A Reaction Orientating reactants correctly in space for. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. How to amazons lower the activation energy of chemical reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The activation energy can be thought. Will Lower The Activation Energy For A Reaction.
From www.cheric.org
Chemical Reaction (Reaction rate) Will Lower The Activation Energy For A Reaction It is obtained by measuring a reaction rate constant at different temperatures. How to amazons lower the activation energy of chemical reaction? Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to. Will Lower The Activation Energy For A Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. Select all that apply a.. Will Lower The Activation Energy For A Reaction.
From www.expii.com
Energy Diagram — Overview & Parts Expii Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The problem is that the activation energy is not derived the way you think. If the. Will Lower The Activation Energy For A Reaction.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Will Lower The Activation Energy For A Reaction How to amazons lower the activation energy of chemical reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Orientating reactants correctly in space for. If a reaction has a low activation energy. In chemistry, activation energy is the minimum amount of energy required for a. Will Lower The Activation Energy For A Reaction.
From www.slideserve.com
PPT Activation Energy … PowerPoint Presentation, free download ID Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. The problem is that the activation energy is not. Will Lower The Activation Energy For A Reaction.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free Will Lower The Activation Energy For A Reaction Orientating reactants correctly in space for. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. It is obtained. Will Lower The Activation Energy For A Reaction.
From www.numerade.com
19. The potential energy diagram below represents a reaction. B 6 1 Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. Orientating reactants correctly in space for. It is obtained by measuring a reaction rate constant at different temperatures. If a reaction has a low activation energy. Of the given choices, adding. Will Lower The Activation Energy For A Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Will Lower The Activation Energy For A Reaction The problem is that the activation energy is not derived the way you think. How to amazons lower the activation energy of chemical reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In chemistry, activation energy is the minimum amount of energy required for a. Will Lower The Activation Energy For A Reaction.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Will Lower The Activation Energy For A Reaction Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. How to amazons lower the activation energy of chemical reaction? The activation energy can be. Will Lower The Activation Energy For A Reaction.
From fs.blog
Activation Energy Why Getting Started Is the Hardest Part Will Lower The Activation Energy For A Reaction The problem is that the activation energy is not derived the way you think. It is obtained by measuring a reaction rate constant at different temperatures. Select all that apply a. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In chemistry, activation energy is the. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved This is an energy diagram illustrating a chemical Will Lower The Activation Energy For A Reaction Select all that apply a. If a reaction has a low activation energy. Orientating reactants correctly in space for. The problem is that the activation energy is not derived the way you think. How to amazons lower the activation energy of chemical reaction? In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Of the. Will Lower The Activation Energy For A Reaction.
From yuremnewshuynh.blogspot.com
Calculate the Activation Energy for the Reaction 2nocl Will Lower The Activation Energy For A Reaction Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. The problem is that the activation energy is not derived the way you think. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. If the activation energy is high for a reaction,. Will Lower The Activation Energy For A Reaction.
From scienceinfo.com
Activation Energy Definition, Unit, Formula, Calculations Will Lower The Activation Energy For A Reaction It is obtained by measuring a reaction rate constant at different temperatures. If a reaction has a low activation energy. The problem is that the activation energy is not derived the way you think. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and. Will Lower The Activation Energy For A Reaction.
From www.coursehero.com
[Solved] b. What letter in the figure represents the activation energy Will Lower The Activation Energy For A Reaction Select all that apply a. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. The problem is that the activation energy is not derived the way you think. If a reaction has a low activation energy. If the activation energy. Will Lower The Activation Energy For A Reaction.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Will Lower The Activation Energy For A Reaction How to amazons lower the activation energy of chemical reaction? Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Orientating reactants correctly in space. Will Lower The Activation Energy For A Reaction.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting Will Lower The Activation Energy For A Reaction Orientating reactants correctly in space for. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. It is obtained by measuring a reaction rate constant at different temperatures. The problem is that the activation energy is not derived the way you think. If the activation energy is high for a reaction, then only a few. Will Lower The Activation Energy For A Reaction.
From byjus.com
the energy of activation of a forward reaction is 60 kcal the energy of Will Lower The Activation Energy For A Reaction The problem is that the activation energy is not derived the way you think. If a reaction has a low activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Select all that apply a. In chemistry, activation energy is the minimum amount of energy. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved Based on this diagram, why would a lower activation Will Lower The Activation Energy For A Reaction How to amazons lower the activation energy of chemical reaction? The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If a reaction has a low activation energy. Orientating reactants correctly in space for. If the activation energy is high for. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved The following figure shows molecular energy Will Lower The Activation Energy For A Reaction The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If a reaction has a low activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to.. Will Lower The Activation Energy For A Reaction.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 3.2.4 Activation Energy翰林国际教育 Will Lower The Activation Energy For A Reaction It is obtained by measuring a reaction rate constant at different temperatures. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. Select all that apply a. If a reaction has a low activation energy. The activation energy can be thought of as the. Will Lower The Activation Energy For A Reaction.
From brainly.com
catalysts are molecules that lower the activation energy for a given Will Lower The Activation Energy For A Reaction Select all that apply a. If the activation energy is high for a reaction, then only a few particles will have enough energy to collide so the reaction will be slow. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products.. Will Lower The Activation Energy For A Reaction.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC Will Lower The Activation Energy For A Reaction How to amazons lower the activation energy of chemical reaction? It is obtained by measuring a reaction rate constant at different temperatures. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. Orientating reactants correctly in space for. The problem is that the activation energy is not derived. Will Lower The Activation Energy For A Reaction.
From stewart-switch.com
Label The Energy Diagram For A Twostep Reaction Will Lower The Activation Energy For A Reaction Select all that apply a. The problem is that the activation energy is not derived the way you think. How to amazons lower the activation energy of chemical reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In chemistry, activation energy is the minimum amount. Will Lower The Activation Energy For A Reaction.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Will Lower The Activation Energy For A Reaction In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The activation energy can be thought of as the magnitude of a potential barrier that the reacting molecules need to overcome to initiate a reaction and convert into products. If the activation energy is high for a. Will Lower The Activation Energy For A Reaction.
From socratic.org
What are activation energies? Socratic Will Lower The Activation Energy For A Reaction It is obtained by measuring a reaction rate constant at different temperatures. Select all that apply a. Orientating reactants correctly in space for. How to amazons lower the activation energy of chemical reaction? Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. The problem is that the. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved Activation energy is the energy that a reaction must Will Lower The Activation Energy For A Reaction The problem is that the activation energy is not derived the way you think. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. It is obtained by measuring a reaction rate. Will Lower The Activation Energy For A Reaction.
From www.chegg.com
Solved Of the following, _______ will lower the activation Will Lower The Activation Energy For A Reaction In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Orientating reactants correctly in space for. If a reaction has a low activation energy. Of the given choices, adding a catalyst for the reaction is the one that will lower the activation energy of a reaction. How. Will Lower The Activation Energy For A Reaction.