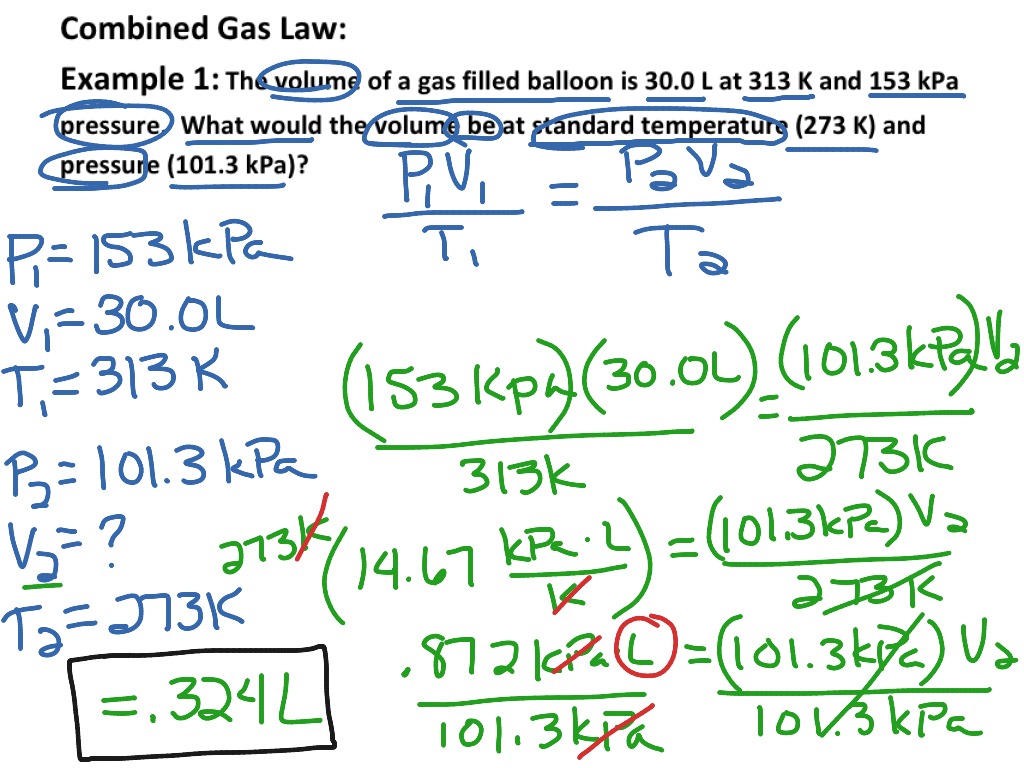
Combined Gas Law Formula Derivation . The most common form of the equation for the combined gas law is as follows: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. Derivation of the combined gas law. At constant temperature a volume gas is inversely proportionally to applied pressure this is. P is the pressure of the gas. $\begingroup$ according to boyle's law: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. The combined gas law is a formula about ideal gases. August 18, 2023 august 15, 2023 by jyoti bashyal. T is the temperature of the gas. It comes from putting together three different laws about the pressure, volume,. The combined gas law is a combination of three gas laws:.
from www.showme.com
At constant temperature a volume gas is inversely proportionally to applied pressure this is. P is the pressure of the gas. Derivation of the combined gas law. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. The combined gas law is a combination of three gas laws:. $\begingroup$ according to boyle's law: The combined gas law is a formula about ideal gases. The most common form of the equation for the combined gas law is as follows: It comes from putting together three different laws about the pressure, volume,.
ShowMe derivation of combined gas law
Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. August 18, 2023 august 15, 2023 by jyoti bashyal. The combined gas law is a combination of three gas laws:. P is the pressure of the gas. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law is a formula about ideal gases. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. T is the temperature of the gas. It comes from putting together three different laws about the pressure, volume,. The most common form of the equation for the combined gas law is as follows: Derivation of the combined gas law. $\begingroup$ according to boyle's law:
From mappingmemories.ca
pálido Claraboya Superar r gases ideales Patentar Destilar Lidiar con Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. Derivation of the combined gas law. August 18, 2023 august 15, 2023 by jyoti bashyal. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. T is the temperature of the gas. The combined gas law is a formula. Combined Gas Law Formula Derivation.
From mmerevise.co.uk
The Ideal Gas Equation MME Combined Gas Law Formula Derivation The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. August 18, 2023 august 15, 2023 by jyoti bashyal. The most common form of the equation for the combined gas law is as follows: $\begingroup$ according to boyle's law: P is the pressure of the gas. Derivation of the combined gas. Combined Gas Law Formula Derivation.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills Combined Gas Law Formula Derivation $\begingroup$ according to boyle's law: Derivation of the combined gas law. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law is a combination of three gas laws:. It comes from putting together three different laws about the pressure, volume,. August 18, 2023 august 15, 2023 by jyoti bashyal. The combined gas. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Combined Gas Law Formula Derivation The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The combined gas law is a formula about ideal gases. T is the temperature of the gas. August 18, 2023 august 15, 2023 by jyoti bashyal. At constant temperature a volume gas. Combined Gas Law Formula Derivation.
From mydigitalkemistry.com
Ideal gas law derivation Combined Gas Laws Gases Best Online Free Combined Gas Law Formula Derivation The most common form of the equation for the combined gas law is as follows: $\begingroup$ according to boyle's law: T is the temperature of the gas. August 18, 2023 august 15, 2023 by jyoti bashyal. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the. Combined Gas Law Formula Derivation.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Combined Gas Law Formula Derivation P is the pressure of the gas. T is the temperature of the gas. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. It comes from putting together three different laws about the pressure, volume,. The most common form of the. Combined Gas Law Formula Derivation.
From www.youtube.com
Deriving the combined and Ideal gas Laws (part 2) YouTube Combined Gas Law Formula Derivation August 18, 2023 august 15, 2023 by jyoti bashyal. P is the pressure of the gas. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. Derivation of the combined gas law. At constant temperature a volume gas is inversely proportionally to. Combined Gas Law Formula Derivation.
From www.showme.com
ShowMe derivation of combined gas law Combined Gas Law Formula Derivation Derivation of the combined gas law. It comes from putting together three different laws about the pressure, volume,. The combined gas law is a formula about ideal gases. The combined gas law is a combination of three gas laws:. T is the temperature of the gas. The most common form of the equation for the combined gas law is as. Combined Gas Law Formula Derivation.
From www.youtube.com
Derivation of of Gas Equation YouTube Combined Gas Law Formula Derivation The combined gas law is a formula about ideal gases. $\begingroup$ according to boyle's law: It comes from putting together three different laws about the pressure, volume,. The most common form of the equation for the combined gas law is as follows: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Formula Derivation At constant temperature a volume gas is inversely proportionally to applied pressure this is. It comes from putting together three different laws about the pressure, volume,. $\begingroup$ according to boyle's law: Derivation of the combined gas law. P is the pressure of the gas. The ideal gas law can also be used to calculate the density of a gas if. Combined Gas Law Formula Derivation.
From www.showme.com
ShowMe derivation of combined gas law Combined Gas Law Formula Derivation $\begingroup$ according to boyle's law: The most common form of the equation for the combined gas law is as follows: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The combined gas law is a formula about ideal gases. P is. Combined Gas Law Formula Derivation.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Combined Gas Law Formula Derivation The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law is a combination of three gas laws:. It comes from putting together three different. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Formula Derivation At constant temperature a volume gas is inversely proportionally to applied pressure this is. It comes from putting together three different laws about the pressure, volume,. The combined gas law is a combination of three gas laws:. August 18, 2023 august 15, 2023 by jyoti bashyal. Derivation of the combined gas law. The combined gas law expresses the relationship between. Combined Gas Law Formula Derivation.
From www.youtube.com
Ideal Gas Laws 2 The Combined Gas Equation YouTube Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. T is the temperature of the gas. At constant temperature a volume gas is inversely proportionally to applied pressure this is. August 18, 2023 august 15, 2023 by jyoti. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Combined Gas Law Formula Derivation P is the pressure of the gas. T is the temperature of the gas. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The combined gas law. Combined Gas Law Formula Derivation.
From studyschoolsgraffito.z21.web.core.windows.net
Gas Laws Equations Sheet Combined Gas Law Formula Derivation At constant temperature a volume gas is inversely proportionally to applied pressure this is. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. $\begingroup$ according to boyle's law: The combined gas law is a combination of three gas laws:. August 18,. Combined Gas Law Formula Derivation.
From studylib.net
GAS LAWS Equation Sheet Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. At constant temperature a volume gas is inversely proportionally to applied pressure this is. Derivation of the combined gas law.. Combined Gas Law Formula Derivation.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. $\begingroup$ according to boyle's law: The most common form of the equation for the combined gas law is as follows: The combined gas law is a formula about ideal gases. At constant temperature a volume gas is inversely proportionally to applied pressure this is. P is the pressure. Combined Gas Law Formula Derivation.
From learningclignensembleu9.z22.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Formula Derivation Derivation of the combined gas law. The most common form of the equation for the combined gas law is as follows: August 18, 2023 august 15, 2023 by jyoti bashyal. It comes from putting together three different laws about the pressure, volume,. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law. Combined Gas Law Formula Derivation.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills Combined Gas Law Formula Derivation $\begingroup$ according to boyle's law: The combined gas law is a formula about ideal gases. T is the temperature of the gas. The combined gas law is a combination of three gas laws:. Derivation of the combined gas law. The most common form of the equation for the combined gas law is as follows: August 18, 2023 august 15, 2023. Combined Gas Law Formula Derivation.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Combined Gas Law Formula Derivation $\begingroup$ according to boyle's law: It comes from putting together three different laws about the pressure, volume,. The most common form of the equation for the combined gas law is as follows: At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT Gas Law Notes Chemistry Semester II PowerPoint Presentation, free Combined Gas Law Formula Derivation It comes from putting together three different laws about the pressure, volume,. P is the pressure of the gas. T is the temperature of the gas. $\begingroup$ according to boyle's law: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Formula Derivation The combined gas law is a formula about ideal gases. Derivation of the combined gas law. P is the pressure of the gas. August 18, 2023 august 15, 2023 by jyoti bashyal. The most common form of the equation for the combined gas law is as follows: The ideal gas law can also be used to calculate the density of. Combined Gas Law Formula Derivation.
From brainly.com
Which equation represents the combined gas law? Combined Gas Law Formula Derivation T is the temperature of the gas. $\begingroup$ according to boyle's law: The combined gas law is a formula about ideal gases. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. The combined gas law is a combination of three gas. Combined Gas Law Formula Derivation.
From mavink.com
Derive Ideal Gas Equation Combined Gas Law Formula Derivation The combined gas law is a formula about ideal gases. Derivation of the combined gas law. $\begingroup$ according to boyle's law: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. P is the pressure of the gas. The most common form. Combined Gas Law Formula Derivation.
From www.showme.com
ShowMe derivation of combined gas law Combined Gas Law Formula Derivation Derivation of the combined gas law. August 18, 2023 august 15, 2023 by jyoti bashyal. The combined gas law is a formula about ideal gases. $\begingroup$ according to boyle's law: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. T is. Combined Gas Law Formula Derivation.
From www.vrogue.co
Combined Gas Law Definition Formula Examples vrogue.co Combined Gas Law Formula Derivation P is the pressure of the gas. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. It comes from putting together three. Combined Gas Law Formula Derivation.
From brainly.in
derive the ideal gas equation. Brainly.in Combined Gas Law Formula Derivation The combined gas law is a formula about ideal gases. August 18, 2023 august 15, 2023 by jyoti bashyal. It comes from putting together three different laws about the pressure, volume,. At constant temperature a volume gas is inversely proportionally to applied pressure this is. P is the pressure of the gas. The most common form of the equation for. Combined Gas Law Formula Derivation.
From studylib.net
GAS LAWS Equation Sheet Combined Gas Law Formula Derivation Derivation of the combined gas law. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law expresses the relationship between the pressure, volume, and. Combined Gas Law Formula Derivation.
From www.sliderbase.com
Gas Laws Presentation Chemistry Combined Gas Law Formula Derivation $\begingroup$ according to boyle's law: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. It comes from putting together three different laws about the pressure, volume,. T is the temperature of the gas. Derivation of the combined gas law. The ideal gas law can also be used to calculate the. Combined Gas Law Formula Derivation.
From scienceinfo.com
Combined Gas Law Formula, Derivation, Examples Combined Gas Law Formula Derivation The combined gas law is a combination of three gas laws:. It comes from putting together three different laws about the pressure, volume,. P is the pressure of the gas. $\begingroup$ according to boyle's law: The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar. Combined Gas Law Formula Derivation.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Formula Derivation At constant temperature a volume gas is inversely proportionally to applied pressure this is. $\begingroup$ according to boyle's law: T is the temperature of the gas. It comes from putting together three different laws about the pressure, volume,. P is the pressure of the gas. The combined gas law is a combination of three gas laws:. The ideal gas law. Combined Gas Law Formula Derivation.
From www.youtube.com
Combined Gas Law Formula (Chemistry) YouTube Combined Gas Law Formula Derivation The combined gas law is a combination of three gas laws:. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an. P is the pressure of the gas. T is the temperature of the gas. $\begingroup$ according to boyle's law: At constant. Combined Gas Law Formula Derivation.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Combined Gas Law Formula Derivation August 18, 2023 august 15, 2023 by jyoti bashyal. It comes from putting together three different laws about the pressure, volume,. At constant temperature a volume gas is inversely proportionally to applied pressure this is. The combined gas law is a formula about ideal gases. Derivation of the combined gas law. The ideal gas law can also be used to. Combined Gas Law Formula Derivation.