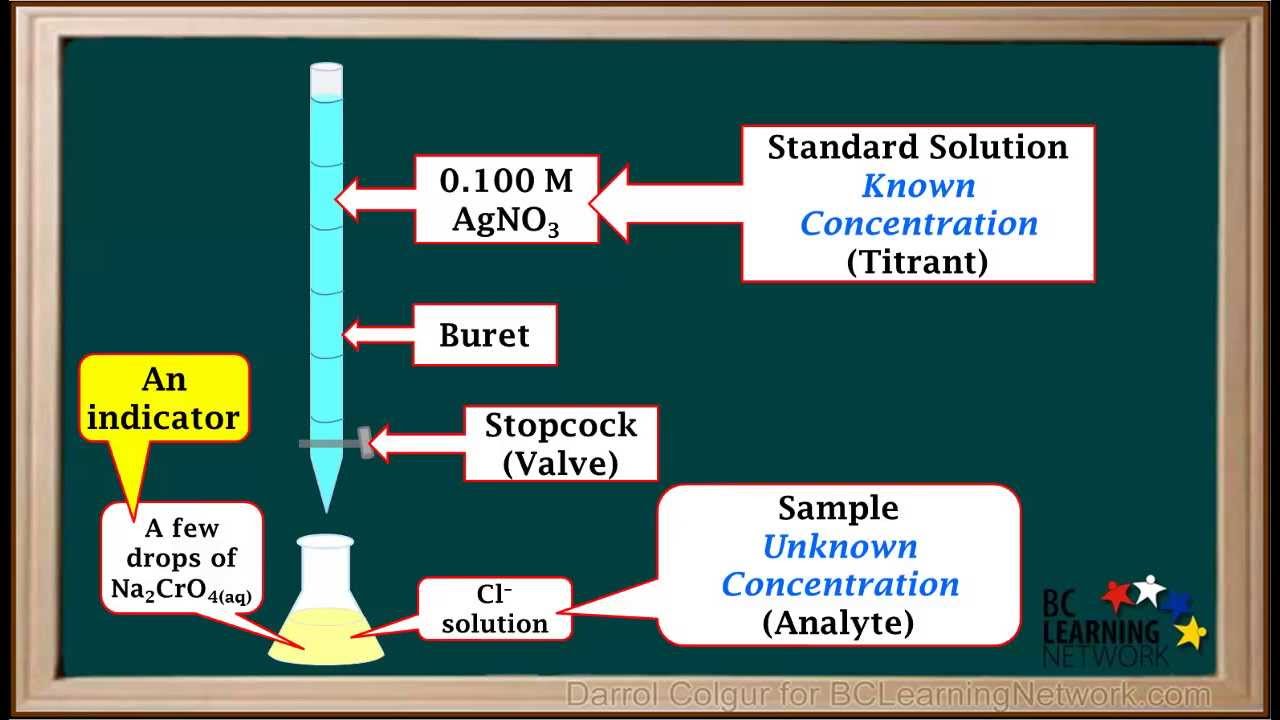
Titration Reaction Equation Example . In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. This equation relates the ph,. Titration is often used to determine the concentration of a solution. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
from www.youtube.com
In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is often used to determine the concentration of a solution. The example below demonstrates the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This equation relates the ph,. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube
Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. The example below demonstrates the. Titration is often used to determine the concentration of a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. This equation relates the ph,. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Reaction Equation Example The example below demonstrates the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. This equation relates the ph,. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In many cases it is not a simple matter. Titration Reaction Equation Example.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Reaction Equation Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find an. Titration Reaction Equation Example.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Reaction Equation Example The example below demonstrates the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. This equation relates the ph,. The above equation works only for neutralizations in which. Titration Reaction Equation Example.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Reaction Equation Example In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is often used to determine the concentration of a solution. Titration is the slow. Titration Reaction Equation Example.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. This equation relates the ph,. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.. Titration Reaction Equation Example.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. In many cases it is not a simple matter to obtain a pure substance, weigh it. Titration Reaction Equation Example.
From ar.inspiredpencil.com
Titration Equation Titration Reaction Equation Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is often used to determine the concentration of a solution.. Titration Reaction Equation Example.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry Titration Reaction Equation Example In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This equation relates the ph,. Titration is often used to determine the concentration of a solution. A titration is used to find an. Titration Reaction Equation Example.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Reaction Equation Example A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In many cases it is not a simple matter to obtain a pure substance, weigh. Titration Reaction Equation Example.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is often used to determine the concentration of a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, 25.0 cm. Titration Reaction Equation Example.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. A titration is. Titration Reaction Equation Example.
From www.numerade.com
SOLVED Write the equation for the titration of Ca2+ with EDTA. Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration Reaction Equation Example.
From www.youtube.com
Titration Calculations YouTube Titration Reaction Equation Example A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. This equation relates the ph,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, 25.0 cm 3 of 0.100 mol/dm 3. Titration Reaction Equation Example.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Reaction Equation Example In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. This equation relates the ph,. In many cases it is not a simple matter to. Titration Reaction Equation Example.
From www.youtube.com
Calculating pH During Titration Reactions YouTube Titration Reaction Equation Example The example below demonstrates the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. Titration is often used to determine the concentration of a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Reaction Equation Example.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This equation relates the ph,. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In many cases it is not a simple matter to obtain a pure substance,. Titration Reaction Equation Example.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Reaction Equation Example This equation relates the ph,. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find. Titration Reaction Equation Example.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Reaction Equation Example This equation relates the ph,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. A titration is used to find an. Titration Reaction Equation Example.
From www.numerade.com
SOLVEDOne way to follow the progress of a titration and detect its Titration Reaction Equation Example This equation relates the ph,. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Titration Reaction Equation Example.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Reaction Equation Example The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This equation relates the ph,. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution. Titration Reaction Equation Example.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Reaction Equation Example In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In many cases it is not a simple matter to obtain a pure substance, weigh it. Titration Reaction Equation Example.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, 25.0. Titration Reaction Equation Example.
From ar.inspiredpencil.com
Titration Equation Titration Reaction Equation Example In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Reaction Equation Example.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration Reaction Equation Example A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The example below demonstrates the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In many cases it is not a simple matter to. Titration Reaction Equation Example.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is used to find an unknown concentration of a solution by reacting it with. Titration Reaction Equation Example.
From www.numerade.com
SOLVED Part Bi Data Analysis of the Titration of a Polybasic Salt Titration Reaction Equation Example The example below demonstrates the. Titration is often used to determine the concentration of a solution. This equation relates the ph,. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. Titration is the slow addition of one solution of a known concentration (called a titrant). Titration Reaction Equation Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Reaction Equation Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is used to find an unknown concentration of a solution by reacting it with. Titration Reaction Equation Example.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration Reaction Equation Example A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly neutralised close neutralisation the reaction between an acid and. In many cases it is not a simple matter to obtain a pure substance, weigh. Titration Reaction Equation Example.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The example below demonstrates the. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. The above equation works only for. Titration Reaction Equation Example.
From mungfali.com
Acid Base Titration Calculation Titration Reaction Equation Example The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This equation relates the ph,. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. Titration is often used to determine the concentration of a solution. In a titration, 25.0 cm 3 of. Titration Reaction Equation Example.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Reaction Equation Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. Titration is often used to determine the concentration of a solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known. Titration Reaction Equation Example.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Titration Reaction Equation Example The example below demonstrates the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This equation relates the ph,. The above equation works only. Titration Reaction Equation Example.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Reaction Equation Example This equation relates the ph,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In many cases it is. Titration Reaction Equation Example.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Reaction Equation Example Titration is often used to determine the concentration of a solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately,. This equation relates the ph,. The example below demonstrates the. Titration. Titration Reaction Equation Example.
From chemistry.stackexchange.com
homework How to calculate the dissociation constant of a weak acid Titration Reaction Equation Example This equation relates the ph,. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. Titration is often used to. Titration Reaction Equation Example.