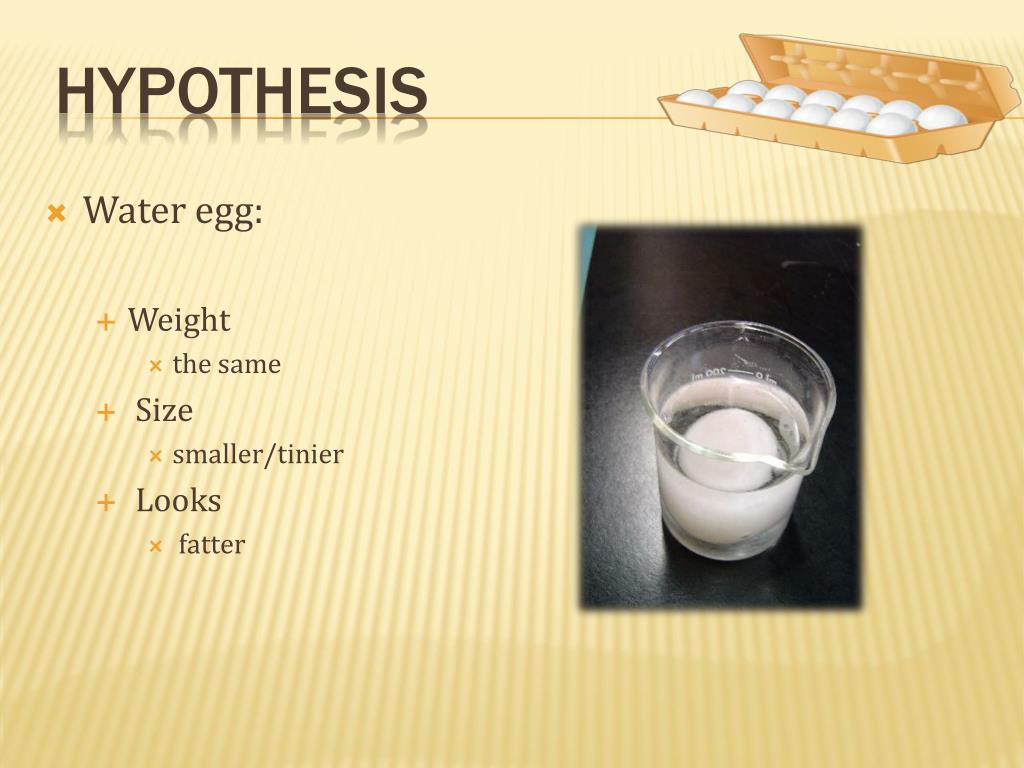
Egg Osmosis Lab Hypothesis . In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The egg shell is made of calcium carbonate and vinegar contains acetic acid. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The outward movement of water causes the egg to shrivel. These two can react to produce calcium acetate and.
from www.slideserve.com
In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. These two can react to produce calcium acetate and. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The egg shell is made of calcium carbonate and vinegar contains acetic acid. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The outward movement of water causes the egg to shrivel.
PPT Egg Osmosis Experiment PowerPoint Presentation, free download
Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. These two can react to produce calcium acetate and. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The outward movement of water causes the egg to shrivel. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The egg shell is made of calcium carbonate and vinegar contains acetic acid. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water.
From learninghypothesis.com
Osmosis Egg Experiment. Handson Osmosis Lab. Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The outward movement of water causes the egg to shrivel. The substance, syrup, which has a higher solute concentration than the. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Nicole's Egg Osmosis Experiment Egg Osmosis Lab Hypothesis In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The egg shell is made of calcium carbonate and vinegar contains acetic acid. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Kim's Egg osmosis experiment Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The outward movement of. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water.. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Marlo's Egg osmosis experiment Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The egg shell is made of calcium carbonate and vinegar contains acetic acid. The outward movement of water causes the egg to shrivel. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The purpose. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The egg shell is made of calcium carbonate and vinegar contains acetic acid. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The substance,. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The outward movement of water causes the egg to shrivel. To reach. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Charisse's Egg osmosis experiment Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. To reach. Egg Osmosis Lab Hypothesis.
From learningschoolexecutry.z14.web.core.windows.net
Egg And Osmosis Lab Egg Osmosis Lab Hypothesis The outward movement of water causes the egg to shrivel. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. To reach. Egg Osmosis Lab Hypothesis.
From dokumen.tips
(PDF) Egg Osmosis and Diffusion Experiment …€¦ · Egg Osmosis and Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The outward movement of water causes the egg to shrivel. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The purpose of this experiment is to observe an egg as. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. To reach. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Egg osmosis experiment Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The egg shell is made of calcium carbonate and vinegar contains acetic acid. The outward movement of water causes the egg to shrivel. Since the eggs are placed in. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The purpose of this experiment is to observe an egg as a model to understand the concept. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The substance,. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water.. Egg Osmosis Lab Hypothesis.
From worksheetdbointed.z13.web.core.windows.net
Egg Osmosis Experiment Results Measurements Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. Since the eggs are placed in solvents with different levels of sucrose concentration,. Egg Osmosis Lab Hypothesis.
From asciencevideo.blogspot.com
Scientific Videos Osmosis Practical with Eggs Egg Osmosis Lab Hypothesis The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. Since the eggs are placed in solvents with different levels. Egg Osmosis Lab Hypothesis.
From pt.slideshare.net
Egg osmosis experiment Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. These two can react to produce calcium acetate and. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Alex's Egg osmosis Experiment Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The egg shell is made of calcium carbonate and vinegar contains acetic acid. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. In the syrup solution, there will be a. Egg Osmosis Lab Hypothesis.
From www.pinterest.com
EGG Osmosis LAB Osmosis, Science activities, Hypothesis Egg Osmosis Lab Hypothesis In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. These two can react to produce calcium acetate and. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Nathans Egg osmosis experiment Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The outward movement of water causes the egg to shrivel. The egg shell is made of calcium carbonate and vinegar contains acetic acid. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn. Egg Osmosis Lab Hypothesis.
From lessonlistblastoderm.z4.web.core.windows.net
Osmosis Egg Experiment Salt Water Egg Osmosis Lab Hypothesis In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The outward movement of water causes the egg to shrivel. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight. Egg Osmosis Lab Hypothesis.
From www.edrawmax.com
Osmosis Egg Lab Report EdrawMax EdrawMax Templates Egg Osmosis Lab Hypothesis The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The egg shell is made of calcium carbonate and vinegar contains acetic acid. The outward movement of water causes the egg to shrivel. The purpose of this experiment is to observe an egg as a model to understand. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Serena Egg osmosis experiment Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. These two can react to produce calcium acetate and. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The egg shell is made of. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The substance, syrup, which has a higher solute concentration than the interior of the eggs,. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
Nicole's Egg Osmosis Experiment Egg Osmosis Lab Hypothesis In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The egg shell is made of calcium carbonate and vinegar contains acetic. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. Since the eggs are placed in. Egg Osmosis Lab Hypothesis.
From www.youtube.com
Egg experiment demonstrates osmosis and diffusion YouTube Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. To reach equilibrium, osmosis causes the water molecules to move out. Egg Osmosis Lab Hypothesis.
From www.slideshare.net
The egg osmosis experiment Egg Osmosis Lab Hypothesis The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. The outward movement of water causes the egg to shrivel. The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. To reach equilibrium, osmosis causes the water molecules to. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The purpose of this experiment is to observe an egg as a model to understand the concept of osmosis. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The egg shell is made of calcium carbonate and vinegar contains. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The egg shell is made of calcium carbonate and vinegar contains acetic acid. The substance, syrup, which has a higher solute concentration than the interior of the eggs, will cause water to leave the eggs’. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink),. Egg Osmosis Lab Hypothesis.
From learninghypothesis.com
Osmosis Egg Experiment. Handson Osmosis Lab. Egg Osmosis Lab Hypothesis Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in. The egg shell is. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis These two can react to produce calcium acetate and. The outward movement of water causes the egg to shrivel. Since the eggs are placed in solvents with different levels of sucrose concentration, the egg in the hypertonic solution is expected to lose weight (shrink), while the egg in the. The purpose of this experiment is to observe an egg as. Egg Osmosis Lab Hypothesis.
From www.slideserve.com
PPT Egg Osmosis Experiment PowerPoint Presentation, free download Egg Osmosis Lab Hypothesis The outward movement of water causes the egg to shrivel. To reach equilibrium, osmosis causes the water molecules to move out of the egg and into the corn syrup until both solutions have the same concentration of water. The egg shell is made of calcium carbonate and vinegar contains acetic acid. In the syrup solution, there will be a net. Egg Osmosis Lab Hypothesis.