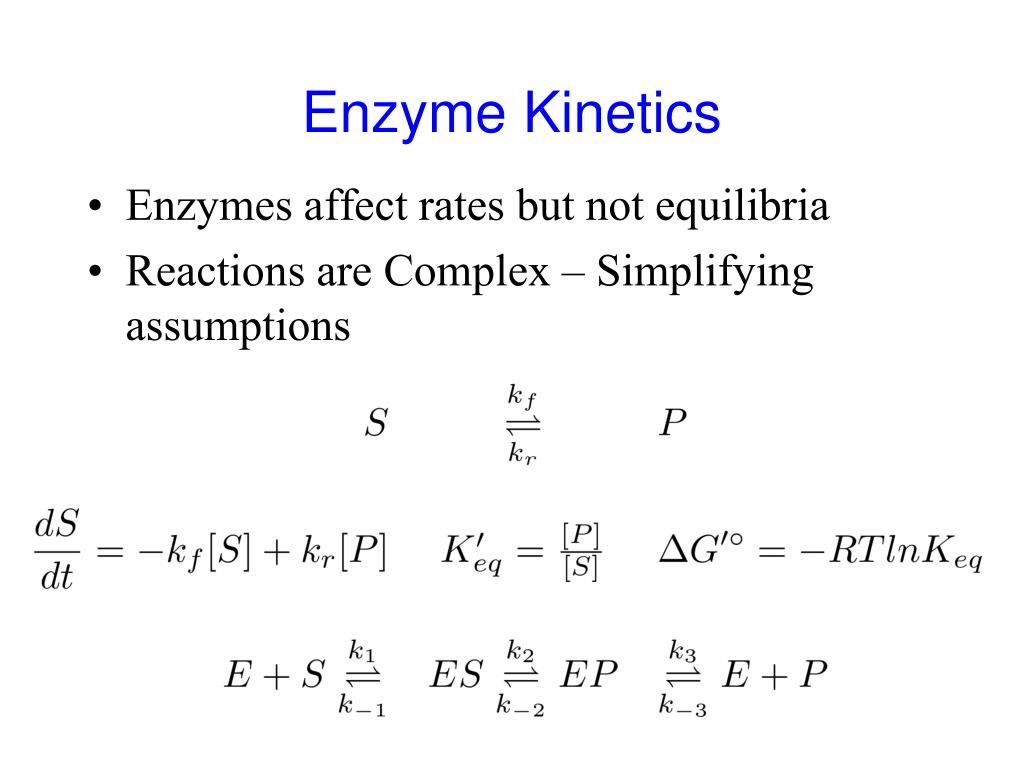
Enzyme Kinetics Equilibrium . in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is followed by. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy.
from www.slideserve.com
That reaction is followed by. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by.
PPT CHAPTER 6 Enzymes PowerPoint Presentation, free download ID815181
Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. That reaction is followed by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such.
From demonstrations.wolfram.com
MichaelisMenten Enzyme and the SteadyState Approximation Enzyme Kinetics Equilibrium you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and. Enzyme Kinetics Equilibrium.
From quizlet.com
Chapter 8 Enzymes Basic Concepts and Diagram Quizlet Enzyme Kinetics Equilibrium you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. That reaction is followed by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of. Enzyme Kinetics Equilibrium.
From bio.libretexts.org
6.2 Enzyme Biology LibreTexts Enzyme Kinetics Equilibrium you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is followed by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics. Enzyme Kinetics Equilibrium.
From www.pinterest.com
Biochemistry 9.2 Enzyme part 1 Enzyme Enzyme Kinetics Equilibrium That reaction is followed by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is followed by. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads. Enzyme Kinetics Equilibrium.
From www.lecturio.com
Enzyme Concise Medical Knowledge Enzyme Kinetics Equilibrium enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is. Enzyme Kinetics Equilibrium.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Enzyme Kinetics Equilibrium That reaction is followed by. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Chapter 8 Enzymes Basic Concepts and PowerPoint Enzyme Kinetics Equilibrium That reaction is followed by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. in this chapter, you will learn the fundamental concepts of chemical and. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics Equilibrium you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is followed by. you have learned previously that enzymes don't change the. Enzyme Kinetics Equilibrium.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. That reaction is followed by. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads. Enzyme Kinetics Equilibrium.
From www.youtube.com
Enzyme rapid equilibrium and steadystate assumptions Topic Enzyme Kinetics Equilibrium That reaction is followed by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. you have learned previously that enzymes don't change the equilibrium. Enzyme Kinetics Equilibrium.
From teachmephysiology.com
Enzyme Structure Function MichaelisMenten Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction. Enzyme Kinetics Equilibrium.
From www.sqadia.com
Enzyme I Introduction Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. you have. Enzyme Kinetics Equilibrium.
From www.researchgate.net
(PDF) The Chemical Master Equation Approach to Nonequilibrium Steady Enzyme Kinetics Equilibrium That reaction is followed by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of. Enzyme Kinetics Equilibrium.
From www.slideshare.net
Enzyme Behavior and Analysis of Rapid Equilibrium and Stead… Enzyme Kinetics Equilibrium enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. That reaction. Enzyme Kinetics Equilibrium.
From www.researchgate.net
Enzyme of DgcR ac Enzymatic progress curves of wildtype DgcR Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. That reaction is followed by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Inhibited Enzyme PowerPoint Presentation, free download Enzyme Kinetics Equilibrium That reaction is followed by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. you have learned previously that enzymes don't change the equilibrium constant for a. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme Study the rate of enzyme catalyzed reactions Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT ENZYME PowerPoint Presentation, free download ID250062 Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. That reaction is followed by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID3195326 Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. you have learned previously that enzymes don't change the equilibrium constant for a reaction,. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme Study the rate of enzyme catalyzed reactions Enzyme Kinetics Equilibrium enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. That reaction is followed by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT ENZYME PowerPoint Presentation, free download ID2288984 Enzyme Kinetics Equilibrium That reaction is followed by. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. enzymes are protein catalysts, they influence the kinetics but. Enzyme Kinetics Equilibrium.
From www.britannica.com
Chemical Definition, Equations, & Facts Britannica Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a. Enzyme Kinetics Equilibrium.
From www.scribd.com
(Enzymes) Chemical Equilibrium Chemical Reactions Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. That reaction is followed by. you have learned previously that enzymes don't change the equilibrium. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT CHAPTER 6 Enzymes PowerPoint Presentation, free download ID815181 Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. for a. Enzyme Kinetics Equilibrium.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is. Enzyme Kinetics Equilibrium.
From www.slideshare.net
Enzyme Enzyme Kinetics Equilibrium enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. That reaction is followed by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction). Enzyme Kinetics Equilibrium.
From quizlet.com
Chapter 9 Enzyme Diagram Quizlet Enzyme Kinetics Equilibrium 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT CHAPTER 6 Enzymes PowerPoint Presentation, free download ID815181 Enzyme Kinetics Equilibrium you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. That reaction is followed by. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. enzymes are protein catalysts, they influence the kinetics but. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Binding and for Experimental Biologists Lecture 5 Enzyme Kinetics Equilibrium enzymes are protein catalysts, they influence the kinetics but not the thermodynamics of a reaction. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore the rate of reaction) remains. That reaction is followed by. in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics Equilibrium in this chapter, you will learn the fundamental concepts of chemical and enzyme catalysis as well as kinetics such. for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and therefore. Enzyme Kinetics Equilibrium.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzyme Kinetics Equilibrium for a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited by. you have learned previously that enzymes don't change the equilibrium constant for a reaction, but rather lowers the activation energy. 10 rows when the reaction reaches equilibrium (steady state phase), the es concentration (and. Enzyme Kinetics Equilibrium.