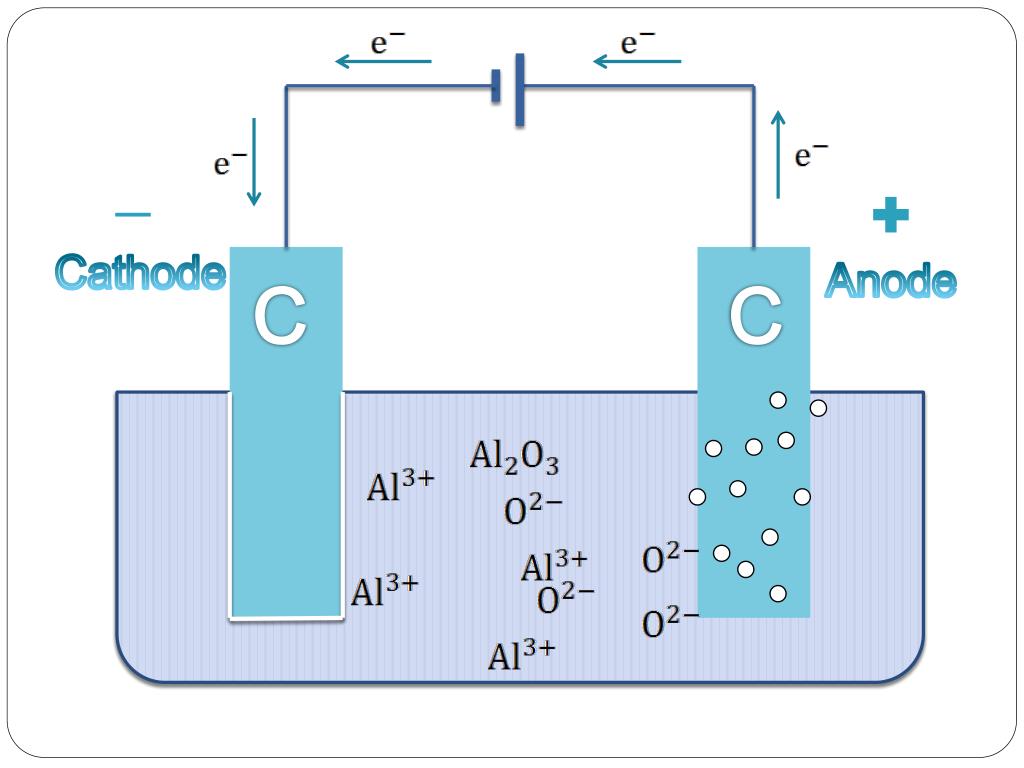
Electrodes Electrolysis Of Aluminium . The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. The extraction is done by. Electrolysis involves using electricity to break down electrolytes to form. Aluminium oxide is then dissolved in molten. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form elements. Alumina is dissolved in the fused. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis and extraction of aluminium extracting aluminium. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. The process of aluminium extraction by electrolysis. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon.
from falibert.blogspot.com
The process of aluminium extraction by electrolysis. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis and extraction of aluminium ionic half equations. The extraction is done by. Electrolysis and extraction of aluminium extracting aluminium. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Alumina is dissolved in the fused. Bauxite is first purified to produce aluminium oxide, al 2 o 3.
Electrolysis Of Aluminium Oxide Extraction of Aluminium by
Electrodes Electrolysis Of Aluminium Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Alumina is dissolved in the fused. Electrolysis and extraction of aluminium ionic half equations. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Electrolysis involves using electricity to break down electrolytes to form elements. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Electrolysis involves using electricity to break down electrolytes to form. The process of aluminium extraction by electrolysis. Electrolysis and extraction of aluminium extracting aluminium. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The extraction is done by. Aluminium oxide is then dissolved in molten. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal.
From www.researchgate.net
Schematic drawing of an aluminum electrolysis cell Download Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form. Electrolysis and extraction of aluminium extracting aluminium. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Alumina is dissolved in the fused. The. Electrodes Electrolysis Of Aluminium.
From www.doubtnut.com
Draw and label the diagram of electrolysis of alumina and explain the Electrodes Electrolysis Of Aluminium The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Electrolysis involves using electricity to break down electrolytes to form. The extraction is done by. Alumina is dissolved in the fused. Electrolysis of the alumina/cryolite solution gives. Electrodes Electrolysis Of Aluminium.
From www.slideserve.com
PPT Do now! PowerPoint Presentation ID2938610 Electrodes Electrolysis Of Aluminium Alumina is dissolved in the fused. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Electrolysis involves using. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Physics Experiment Electrolysis Of Water With Aluminium Graphite Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Aluminium oxide is then dissolved in. Electrodes Electrolysis Of Aluminium.
From arelifersbrock.blogspot.com
Electrolysis of Aluminium Oxide Electrodes Electrolysis Of Aluminium Aluminium oxide is then dissolved in molten. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis and extraction of aluminium extracting aluminium. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. The process of aluminium extraction by electrolysis. Electrolysis and extraction. Electrodes Electrolysis Of Aluminium.
From www.nagwa.com
Question Video Identifying the Reaction That Occurs at the Anode Electrodes Electrolysis Of Aluminium Alumina is dissolved in the fused. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Electrolysis and extraction of aluminium ionic half equations. The process of aluminium extraction by electrolysis. Electrolysis and extraction of aluminium extracting aluminium. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Aluminium is above. Electrodes Electrolysis Of Aluminium.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Electrolysis Of Aluminium Oxide Video Electrodes Electrolysis Of Aluminium Bauxite is first purified to produce aluminium oxide, al 2 o 3. The process of aluminium extraction by electrolysis. Aluminium oxide is then dissolved in molten. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break. Electrodes Electrolysis Of Aluminium.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5931404 Electrodes Electrolysis Of Aluminium Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Alumina is dissolved in the fused. Aluminium oxide is then dissolved in molten. The bauxite is purified to produce aluminium oxide, a white powder. Electrodes Electrolysis Of Aluminium.
From courses.lumenlearning.com
Occurrence and Preparation of the Representative Metals Chemistry Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. The process of aluminium extraction by electrolysis. Electrolysis. Electrodes Electrolysis Of Aluminium.
From electrolysis1.weebly.com
Electrolysis of Aluminium Oxide Electrolysis Electrodes Electrolysis Of Aluminium Alumina is dissolved in the fused. The process of aluminium extraction by electrolysis. Electrolysis and extraction of aluminium ionic half equations. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Aluminium oxide is then dissolved in molten. Aluminium is above carbon in the reactivity series and can therefore not be extracted with. Electrodes Electrolysis Of Aluminium.
From www.alamy.com
Smelting aluminium by electrolysis. Editable labeled diagram Stock Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium extracting aluminium. Aluminium oxide is then dissolved in molten. Electrolysis involves using electricity to break down electrolytes to form elements. The process of aluminium extraction by electrolysis. Alumina is dissolved in the fused. Electrolysis and extraction of aluminium ionic half equations. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium. Electrodes Electrolysis Of Aluminium.
From www.nagwa.com
Question Video Explaining Why Aluminum ore Must be Molten During Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium extracting aluminium. Electrolysis involves using electricity to break down electrolytes to form. Electrolysis and extraction of aluminium ionic half equations. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. The process of aluminium extraction by electrolysis. Electrolysis involves using electricity to break down electrolytes. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
GCSE Chemistry (Science) Electrolysis of Aluminium Question Revision Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis and extraction of aluminium extracting aluminium. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The process of aluminium extraction by electrolysis. The bauxite is. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Aluminum as Electrodes (H2OElectrolysis) YouTube Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium extracting aluminium. The process of aluminium extraction by electrolysis. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Alumina is dissolved in the fused. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. The extraction is done by. Electrolysis and extraction of. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Electrolysis of aluminium Oxide by Pro. Asif Ali Jamali YouTube Electrodes Electrolysis Of Aluminium One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis involves using electricity to break down electrolytes to form. Aluminium oxide is then dissolved in molten. The process of aluminium extraction by electrolysis. The extraction is done by. Bauxite is first purified to produce aluminium oxide, al 2 o. Electrodes Electrolysis Of Aluminium.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Electrodes Electrolysis Of Aluminium One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Electrolysis and extraction of aluminium extracting aluminium. The bauxite is purified to. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Electrolysis of Aluminium Oxide GCSE Chemistry YouTube Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis involves using electricity to break down electrolytes to form elements. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. Electrolysis and extraction of aluminium ionic half equations. Alumina is dissolved. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Physics Experiment Electrolysis Of Water With Aluminium Electrode Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Alumina is dissolved in the fused. The extraction is done by. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. One of the perspective ways is the. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
The Extraction of Aluminium Electrolysis (GCSE Chemistry) YouTube Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium ionic half equations. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Alumina is dissolved in the fused. Electrolysis involves using electricity to break down electrolytes to form elements. Aluminium oxide is then dissolved in molten. The process of aluminium extraction by electrolysis. Electrolysis of the alumina/cryolite solution gives aluminium at the. Electrodes Electrolysis Of Aluminium.
From falibert.blogspot.com
Electrolysis Of Aluminium Oxide Extraction of Aluminium by Electrodes Electrolysis Of Aluminium Aluminium oxide is then dissolved in molten. Alumina is dissolved in the fused. The extraction is done by. Electrolysis involves using electricity to break down electrolytes to form. The process of aluminium extraction by electrolysis. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Bauxite is first purified to produce aluminium oxide, al 2. Electrodes Electrolysis Of Aluminium.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 Electrodes Electrolysis Of Aluminium The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Electrolysis involves using electricity to break down electrolytes to form. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis involves using electricity to break. Electrodes Electrolysis Of Aluminium.
From ppt-online.org
Electrolysis презентация онлайн Electrodes Electrolysis Of Aluminium Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. The process of aluminium extraction by electrolysis. Aluminium oxide is then dissolved in molten. The extraction is done by. Aluminium is above carbon in the reactivity series and can therefore not be extracted. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
How to Teach the Electrolysis of Aluminium YouTube Electrodes Electrolysis Of Aluminium The extraction is done by. Electrolysis involves using electricity to break down electrolytes to form. Electrolysis and extraction of aluminium extracting aluminium. Aluminium oxide is then dissolved in molten. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Aluminium is. Electrodes Electrolysis Of Aluminium.
From question.pandai.org
Extraction of Metals from its Ore Electrodes Electrolysis Of Aluminium One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. The process of aluminium extraction by electrolysis. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. The extraction is done by. Alumina is dissolved in the fused. Aluminium oxide is then dissolved. Electrodes Electrolysis Of Aluminium.
From falibert.blogspot.com
Electrolysis Of Aluminium Oxide Extraction of Aluminium by Electrodes Electrolysis Of Aluminium Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. The process of aluminium extraction by electrolysis. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Alumina is. Electrodes Electrolysis Of Aluminium.
From mungfali.com
Electrolysis Process Diagram Electrodes Electrolysis Of Aluminium Aluminium oxide is then dissolved in molten. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis involves using. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Extraction of Aluminium (Electrolysis of Aluminium Oxide) GCSE Electrodes Electrolysis Of Aluminium One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form elements. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted. Aluminium is above carbon. Electrodes Electrolysis Of Aluminium.
From www.slideserve.com
PPT Secondary 4 Chemistry Extraction of Aluminium via electrolysis JT Electrodes Electrolysis Of Aluminium Bauxite is first purified to produce aluminium oxide, al 2 o 3. Electrolysis involves using electricity to break down electrolytes to form elements. Aluminium oxide is then dissolved in molten. Electrolysis and extraction of aluminium extracting aluminium. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis of the. Electrodes Electrolysis Of Aluminium.
From mavink.com
Electrolysis Cell Diagram Electrodes Electrolysis Of Aluminium Aluminium oxide is then dissolved in molten. Electrolysis involves using electricity to break down electrolytes to form elements. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis and extraction of aluminium extracting aluminium. Electrolysis involves using electricity to break down electrolytes to form. Electrolysis and extraction of aluminium. Electrodes Electrolysis Of Aluminium.
From falibert.blogspot.com
Electrolysis Of Aluminium Oxide Extraction of Aluminium by Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium extracting aluminium. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The extraction is done by. Electrolysis and extraction of aluminium ionic half equations. Aluminium oxide is then dissolved in molten. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form.. Electrodes Electrolysis Of Aluminium.
From www.toppr.com
Write chemical equation the event.Electrolysis of alumina is done. Electrodes Electrolysis Of Aluminium Aluminium oxide is then dissolved in molten. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The extraction is done by. The process of aluminium extraction by electrolysis. Electrolysis and extraction of aluminium ionic half equations. Electrolysis involves using electricity to break down electrolytes to form. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and. Electrodes Electrolysis Of Aluminium.
From www.youtube.com
Electrolysis by Aluminium Electrodes YouTube Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium extracting aluminium. The process of aluminium extraction by electrolysis. Alumina is dissolved in the fused. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of the alumina/cryolite solution gives aluminium at the cathode and oxygen at the anode. Bauxite is first purified to produce aluminium oxide, al 2 o 3. Aluminium is. Electrodes Electrolysis Of Aluminium.
From www.onlinemathlearning.com
Extraction of Aluminium (examples, answers, activities, experiment, videos) Electrodes Electrolysis Of Aluminium Electrolysis involves using electricity to break down electrolytes to form. Electrolysis involves using electricity to break down electrolytes to form elements. Alumina is dissolved in the fused. Aluminium is above carbon in the reactivity series and can therefore not be extracted with carbon. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can be extracted.. Electrodes Electrolysis Of Aluminium.
From www.researchgate.net
The basic process of aluminium electrolysis manufacturing Download Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium ionic half equations. Alumina is dissolved in the fused. One of the perspective ways is the electrolytic production of aluminum at low temperature in cells with vertical or horizontal. Electrolysis involves using electricity to break down electrolytes to form elements. The process of aluminium extraction by electrolysis. Electrolysis of the alumina/cryolite solution gives aluminium at. Electrodes Electrolysis Of Aluminium.
From www.w3schools.blog
Aluminium Extraction W3schools Electrodes Electrolysis Of Aluminium Electrolysis and extraction of aluminium ionic half equations. Aluminium oxide is then dissolved in molten. Alumina is dissolved in the fused. The process of aluminium extraction by electrolysis. Bauxite is first purified to produce aluminium oxide, al 2 o 3. The extraction is done by. The bauxite is purified to produce aluminium oxide, a white powder from which aluminium can. Electrodes Electrolysis Of Aluminium.