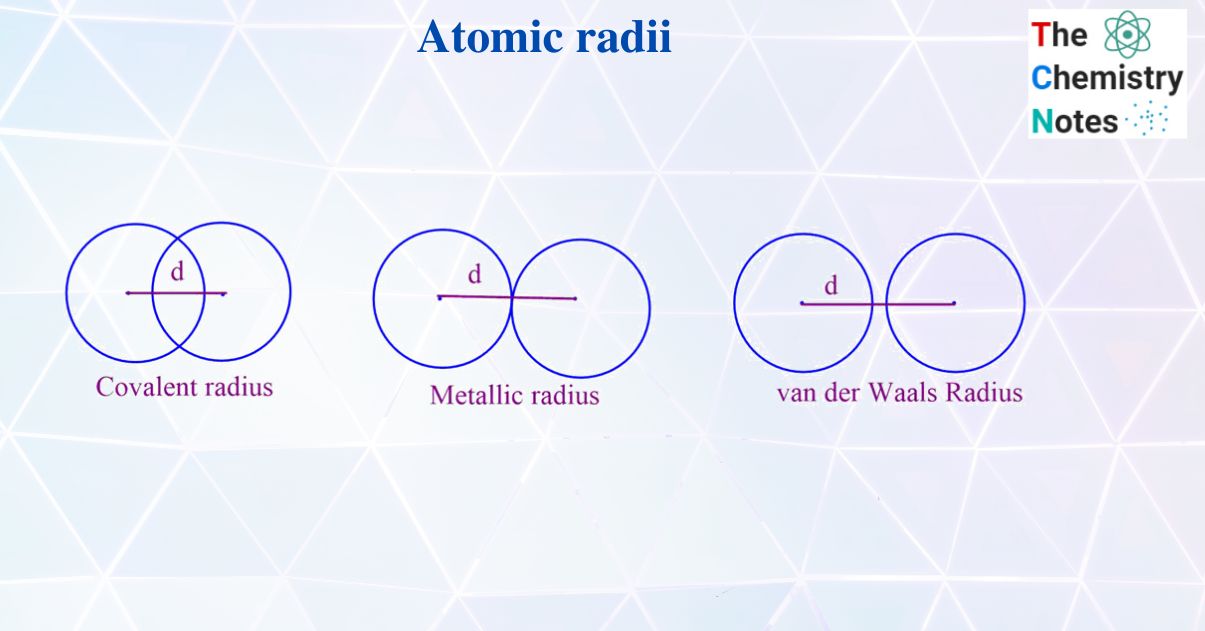
Metallic Radius Definition Class 11 . It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. The metallic radius is the radius of an atom joined by the metallic bond. Since a metal will be a group. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a.
from thechemistrynotes.com
It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. Since a metal will be a group. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is the radius of an atom joined by the metallic bond. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a.
Atomic radius
Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. The metallic radius is the radius of an atom joined by the metallic bond. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. Since a metal will be a group. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined.
From mungfali.com
Atomic Radius Metallic Radius Definition Class 11 Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. Since a metal will be a group. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. (i) metallic radius is half the distance between centres of. Metallic Radius Definition Class 11.
From www.youtube.com
CLASSIFICATION OF ELEMENTS PART 4 ATOMIC RADIUS YouTube Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. Among various ways to determine the radius of the atom , covalent radius , metallic radius ,. Metallic Radius Definition Class 11.
From www.researchgate.net
Metallic radii of atoms in metallic glass structures. Download Table Metallic Radius Definition Class 11 (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged. Metallic Radius Definition Class 11.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2487087 Metallic Radius Definition Class 11 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. Since a metal will be a group. The metallic radius is the radius of an atom joined by the metallic bond. The metallic radius is half of the total distance between the nuclei. Metallic Radius Definition Class 11.
From thechemistrynotes.com
Atomic radius Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. The metallic radius is the radius of an atom joined by the metallic bond. Since a metal will be a group. (i) metallic. Metallic Radius Definition Class 11.
From www.yaclass.in
Atomic Radius and Ionic Radii — lesson. Science State Board, Class 10. Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. 3). Metallic Radius Definition Class 11.
From www.teachoo.com
Example 11 A shotputt is a metallic sphere of radius Examples Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is the radius of an atom joined by the metallic bond. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius. Metallic Radius Definition Class 11.
From animalia-life.club
Atomic Radius Diagram Metallic Radius Definition Class 11 The metallic radius is the radius of an atom joined by the metallic bond. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. Among various ways to. Metallic Radius Definition Class 11.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. Since a metal will be a group. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. A crystal contains positive kernel ions which are arranged in. Metallic Radius Definition Class 11.
From www.differencebetween.com
Difference Between Covalent Radius and Metallic Radius Compare the Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. Since a metal will be a group. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. Among various ways to determine the radius of the atom ,. Metallic Radius Definition Class 11.
From www.youtube.com
Metallic Radius Class 11 YouTube Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. The metallic radius is the radius of an atom joined by the metallic bond. (i) metallic radius is half the distance between. Metallic Radius Definition Class 11.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID2487087 Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is the radius of an atom joined by the metallic bond. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond. Among various. Metallic Radius Definition Class 11.
From www.studocu.com
Types of Atomic Radii Covalent radius, Van der waal radius, Metallic Metallic Radius Definition Class 11 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is the radius of an atom joined by the metallic bond. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by. Metallic Radius Definition Class 11.
From sciencenotes.org
Atomic Radius and Ionic Radius Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is the radius of an atom joined by the metallic bond. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius. Metallic Radius Definition Class 11.
From in.pinterest.com
Atomic radius Ionic radius, Chemistry education, Chemistry Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by metallic bond.. Metallic Radius Definition Class 11.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. The metallic radius is the radius of an atom joined by the metallic bond. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. A. Metallic Radius Definition Class 11.
From chemistry.stackexchange.com
periodic table Comparison between van der Waals radius and metallic Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal. Metallic Radius Definition Class 11.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together. Metallic Radius Definition Class 11.
From solveforum.com
[Solved] Comparison between van der Waals radius and metallic radius Metallic Radius Definition Class 11 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. Since a metal will be a group. It. Metallic Radius Definition Class 11.
From www.youtube.com
XI Chemistry Chapter3 Atomic Radius Covalent,Vanderwall , Metallic Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a.. Metallic Radius Definition Class 11.
From slideplayer.com
Elemental Properties and Patterns ppt download Metallic Radius Definition Class 11 Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. It is defined as half of the intemuclear distance between the two adjacent metal ions. Metallic Radius Definition Class 11.
From www.slideserve.com
PPT Atomic Radius PowerPoint Presentation, free download ID6206201 Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice.. Metallic Radius Definition Class 11.
From eziil.com
Sheet Metal Bend Radius Full Guide Chart Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. Since a metal will be a group. (i) metallic radius is half the distance between. Metallic Radius Definition Class 11.
From www.teachoo.com
Question 2 Metallic spheres of radii 6 cm, 8 cm and 10 cm Metallic Radius Definition Class 11 Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. It is defined as half of the intemuclear. Metallic Radius Definition Class 11.
From www.youtube.com
metallic radius metallic radius class 11 NEET JEE chemistry Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. The metallic radius is the radius of an atom joined by the metallic bond. Since a metal will be a group. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. The metallic. Metallic Radius Definition Class 11.
From byjus.com
15. What is vanderwall radius and metallic radius Metallic Radius Definition Class 11 Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in. Metallic Radius Definition Class 11.
From www.yaclass.in
Atomic Radius and Ionic Radii — lesson. Science State Board, Class 10. Metallic Radius Definition Class 11 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is the radius of an atom joined by the metallic bond. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice.. Metallic Radius Definition Class 11.
From enthu.com
What is the Atomic Radius? EnthuZiastic Metallic Radius Definition Class 11 Among various ways to determine the radius of the atom , covalent radius , metallic radius , ionic radius and vander wall radius is defined. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. A crystal contains positive kernel ions which are. Metallic Radius Definition Class 11.
From proper-cooking.info
Measuring Atomic Radius Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. Among various ways to determine the radius of the atom , covalent radius. Metallic Radius Definition Class 11.
From ar.inspiredpencil.com
Atomic Radius Diagram Metallic Radius Definition Class 11 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. (i) metallic radius is half the distance between centres of nuclei of two atoms of. Metallic Radius Definition Class 11.
From www.youtube.com
ATOMIC RADIUS Covalent Radius, Metallic Radius, Van der Waal's Radius Metallic Radius Definition Class 11 A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is the radius of an atom joined by the metallic bond. Since a metal will be a group. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged. Metallic Radius Definition Class 11.
From www.coursehero.com
[Solved] The metallic radius of an aluminum atom is 143 pm. What is the Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. Since a metal will be a group. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a metallic cluster. (i) metallic radius is half the distance between centres of nuclei of two atoms of. Metallic Radius Definition Class 11.
From byjus.com
what are the difference among metallic , covalent and van der waals Metallic Radius Definition Class 11 Since a metal will be a group. 3) metallic radius a metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. The metallic radius is half of the. Metallic Radius Definition Class 11.
From www.youtube.com
ClassXI Chapter3 Periodicity Metallic Radius Van der Waals Radii Metallic Radius Definition Class 11 It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. Metallic Radius Definition Class 11.
From www.youtube.com
Atomic radius Physics wala Covalent radius Metallic radius Vander Metallic Radius Definition Class 11 The metallic radius is half of the total distance between the nuclei of two adjacent atoms in a. A crystal contains positive kernel ions which are arranged in a pattern which is definite in a sea of mobile electrons which are. (i) metallic radius is half the distance between centres of nuclei of two atoms of metal held together by. Metallic Radius Definition Class 11.