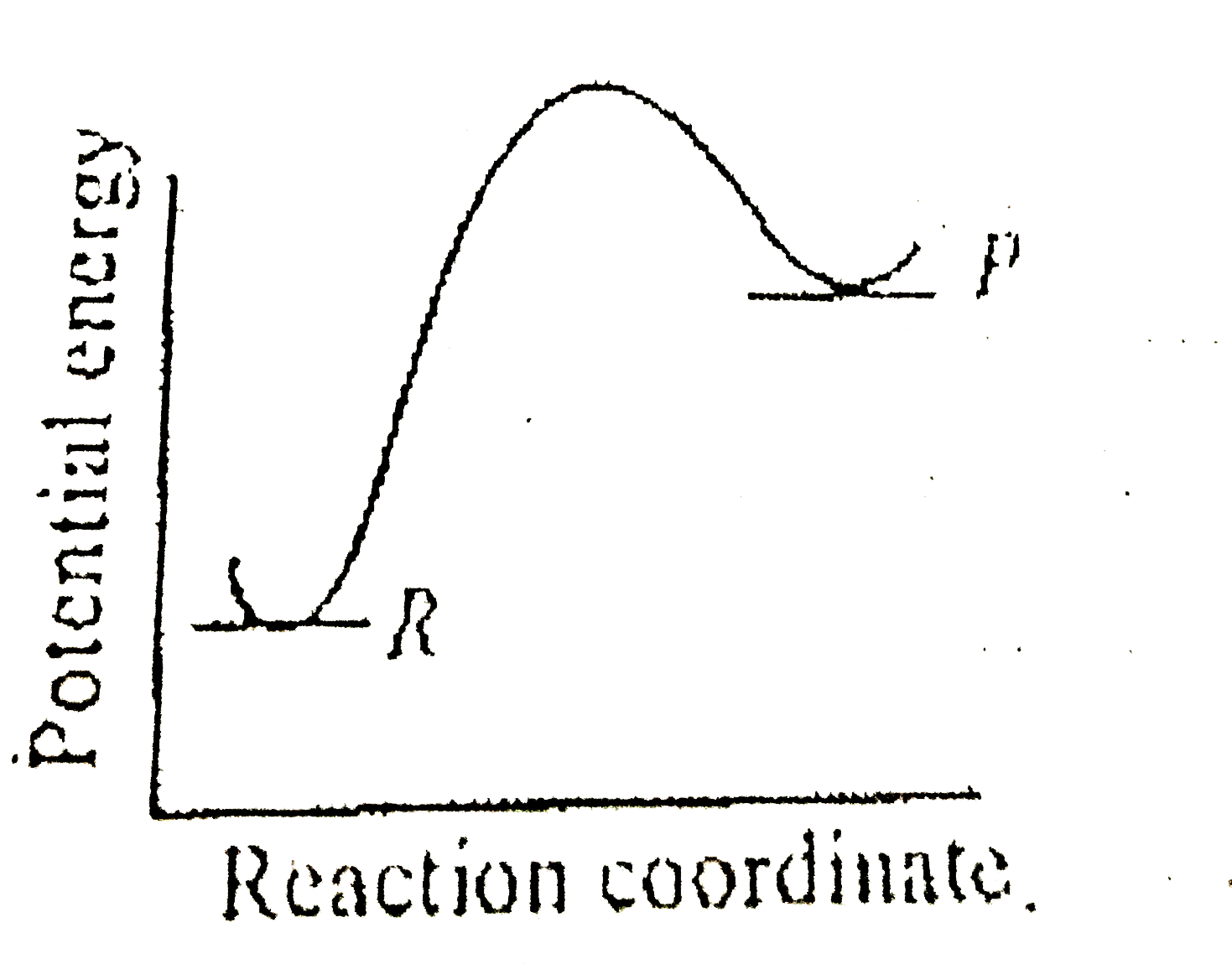
Endothermic Reaction Solution Potassium Chloride . Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. The reaction in question is:. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. When some salts dissolve in water, there is a cooling effect. With potassium chloride, the cooling is. An example of an easy endothermic reaction is dissolving potassium. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. An endothermic reaction is a chemical reaction that absorbs thermal. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions.
from www.doubtnut.com
An endothermic reaction is a chemical reaction that absorbs thermal. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. When some salts dissolve in water, there is a cooling effect. The reaction in question is:. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. An example of an easy endothermic reaction is dissolving potassium. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. With potassium chloride, the cooling is.
An endothermic reaction with high activation energy for the forward re
Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. An example of an easy endothermic reaction is dissolving potassium. An endothermic reaction is a chemical reaction that absorbs thermal. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. When some salts dissolve in water, there is a cooling effect. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. With potassium chloride, the cooling is. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The reaction in question is:.
From www.greelane.com
Exemples de réactions endothermiques Endothermic Reaction Solution Potassium Chloride Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. With potassium chloride, the cooling is. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. When some salts dissolve in water, there is a cooling effect. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. Many endothermic. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Potassium Chloride An endothermic reaction is a chemical reaction that absorbs thermal. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. The reaction in question is:. To understand enthalpies of solution and be able to use them to calculate the heat absorbed. Endothermic Reaction Solution Potassium Chloride.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Solution Potassium Chloride With potassium chloride, the cooling is. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An example of an easy endothermic reaction is dissolving potassium. When some salts dissolve in water, there is a cooling effect. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when. Endothermic Reaction Solution Potassium Chloride.
From www.chegg.com
Solved Part A Exothermic and Endothermic Reactions NH4NO3 Endothermic Reaction Solution Potassium Chloride An example of an easy endothermic reaction is dissolving potassium. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. With potassium chloride, the cooling is. To. Endothermic Reaction Solution Potassium Chloride.
From frugalfun4boys.com
Chilly Chemical Reactions! {Endothermic Reactions} Frugal Fun For Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. The reaction in question is:. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. To understand enthalpies. Endothermic Reaction Solution Potassium Chloride.
From www.chegg.com
Solved when 26.6 of potassium chloride dissolves in 300.0g Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Potassium Chloride When some salts dissolve in water, there is a cooling effect. An endothermic reaction is a chemical reaction that absorbs thermal. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction in question is:. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. When everyday table salt, sodium chloride,. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Potassium Chloride An endothermic reaction is a chemical reaction that absorbs thermal. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. An example of an easy endothermic reaction is dissolving potassium. The reaction in question is:. Many. Endothermic Reaction Solution Potassium Chloride.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. With potassium chloride, the cooling is. When some salts dissolve in water, there is a cooling effect. When everyday table salt, sodium. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Potassium Chloride The reaction in question is:. An example of an easy endothermic reaction is dissolving potassium. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. When some salts dissolve in water, there is a cooling effect. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the. Endothermic Reaction Solution Potassium Chloride.
From www.savemyexams.com
Endothermic & Exothermic Reactions Cambridge O Level Chemistry Endothermic Reaction Solution Potassium Chloride With potassium chloride, the cooling is. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. An endothermic reaction is a chemical. Endothermic Reaction Solution Potassium Chloride.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Solution Potassium Chloride Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. The reaction in question is:. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is. Endothermic Reaction Solution Potassium Chloride.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Solution Potassium Chloride With potassium chloride, the cooling is. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction in question is:. An endothermic reaction is a chemical reaction that absorbs thermal. To understand enthalpies of solution and be able to use them to. Endothermic Reaction Solution Potassium Chloride.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Solution Potassium Chloride An example of an easy endothermic reaction is dissolving potassium. With potassium chloride, the cooling is. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The enthalpies of. Endothermic Reaction Solution Potassium Chloride.
From blog.iceslicer.com
Chloride Spotlight What is Potassium Chloride? Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An example of an easy endothermic reaction is dissolving potassium. When some salts dissolve in water, there is a cooling effect. When. Endothermic Reaction Solution Potassium Chloride.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. With potassium chloride, the cooling is. When some salts dissolve in water, there is a cooling effect. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. An example of. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Potassium Chloride When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. With potassium chloride, the cooling is. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. When some salts dissolve in water, there is a cooling effect. The reaction in question is:. An. Endothermic Reaction Solution Potassium Chloride.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Reaction Solution Potassium Chloride The reaction in question is:. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs thermal. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. With potassium chloride, the cooling is. To understand enthalpies of solution and be able to. Endothermic Reaction Solution Potassium Chloride.
From klasgnqsj.blob.core.windows.net
Endothermic Reaction Graph Example at Britt Fields blog Endothermic Reaction Solution Potassium Chloride Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An example of an easy endothermic reaction is dissolving potassium. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making. Endothermic Reaction Solution Potassium Chloride.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Solution Potassium Chloride When some salts dissolve in water, there is a cooling effect. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. An endothermic reaction is a chemical reaction that absorbs thermal. When everyday table salt, sodium chloride, dissolves in water, the mixture. Endothermic Reaction Solution Potassium Chloride.
From www.numerade.com
SOLVED Use the heat of solution interactive to classify the Endothermic Reaction Solution Potassium Chloride To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. An endothermic reaction is a chemical reaction that absorbs thermal. An example of an easy endothermic reaction is dissolving potassium. With potassium chloride, the cooling is.. Endothermic Reaction Solution Potassium Chloride.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Solution Potassium Chloride When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. With potassium chloride, the cooling is. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs thermal. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. To. Endothermic Reaction Solution Potassium Chloride.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Solution Potassium Chloride Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. The reaction in question is:. An endothermic reaction is a chemical reaction that absorbs thermal. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. When some salts dissolve in water, there is a cooling effect. When everyday table salt,. Endothermic Reaction Solution Potassium Chloride.
From www.toppr.com
A solution of potassium chloride when mixed with silver nitrate Endothermic Reaction Solution Potassium Chloride Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. When some salts dissolve in water, there is a cooling effect. Many. Endothermic Reaction Solution Potassium Chloride.
From slideplayer.com
Energy of Reactions Chemistry ppt download Endothermic Reaction Solution Potassium Chloride With potassium chloride, the cooling is. When some salts dissolve in water, there is a cooling effect. An example of an easy endothermic reaction is dissolving potassium. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or. Endothermic Reaction Solution Potassium Chloride.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Solution Potassium Chloride To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances. Endothermic Reaction Solution Potassium Chloride.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Solution Potassium Chloride Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. The enthalpies of. Endothermic Reaction Solution Potassium Chloride.
From www.nagwa.com
Question Video Determining the Equation That Shows the Reaction at the Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. The reaction in question is:. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. With potassium chloride, the cooling is. An example of an easy endothermic reaction is dissolving potassium. Many. Endothermic Reaction Solution Potassium Chloride.
From mavink.com
Diagram Of Endothermic Reaction Endothermic Reaction Solution Potassium Chloride An endothermic reaction is a chemical reaction that absorbs thermal. With potassium chloride, the cooling is. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. The reaction in question is:. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. When some salts dissolve in water, there is a. Endothermic Reaction Solution Potassium Chloride.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Reaction Solution Potassium Chloride The reaction in question is:. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. An example of an easy endothermic reaction is dissolving potassium. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted. Endothermic Reaction Solution Potassium Chloride.
From www.youtube.com
A solution of potassium chloride when mixed with silver nitrate Endothermic Reaction Solution Potassium Chloride Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. An endothermic reaction is a chemical reaction that absorbs thermal. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances. Endothermic Reaction Solution Potassium Chloride.
From www.slideserve.com
PPT Endothermic Reaction Investigation Ammonium Chloride + Water Endothermic Reaction Solution Potassium Chloride Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. With potassium chloride, the cooling is. Dissolution disrupts the strong electrostatic bonds between the oppositely charged ions of the lattice. Examples of endothermic. Endothermic Reaction Solution Potassium Chloride.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Reaction Solution Potassium Chloride To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. An example of an easy endothermic reaction is dissolving potassium. When everyday table salt, sodium. Endothermic Reaction Solution Potassium Chloride.
From slideplayer.com
Thermochemistry Comprehensive tutorial notes ppt download Endothermic Reaction Solution Potassium Chloride An example of an easy endothermic reaction is dissolving potassium. To understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Many endothermic and exothermic reactions involve toxic chemicals, extreme heat or cold, or messy disposal methods. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Endothermic Reaction Solution Potassium Chloride.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Reaction Solution Potassium Chloride The enthalpies of solution for some salts can be positive values, in these cases the temperatures of the solution decrease as the substances dissolve;. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction in question is:. When everyday table salt, sodium chloride, dissolves in water, the mixture cools slightly. To understand enthalpies of. Endothermic Reaction Solution Potassium Chloride.