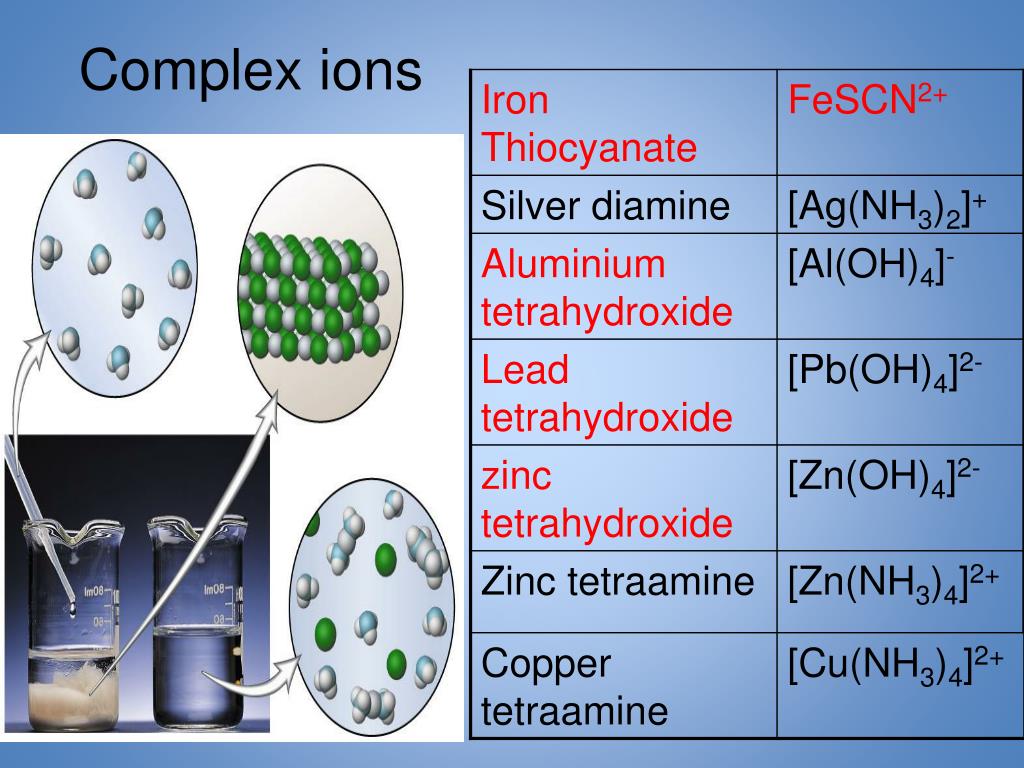
Zinc Ion Ammonia . Zinc +2 ion and dilute ammonia solution reaction. Burns readily in air to form white zno and combines with many nonmetals. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Because it is a stronger.
from www.slideserve.com
Because it is a stronger. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Burns readily in air to form white zno and combines with many nonmetals. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Zinc +2 ion and dilute ammonia solution reaction. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial.
PPT Complex Ions PowerPoint Presentation, free download ID2835720
Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Burns readily in air to form white zno and combines with many nonmetals. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Because it is a stronger. Zinc +2 ion and dilute ammonia solution reaction. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Zinc Ion Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). As an example of the formation of complex. Zinc Ion Ammonia.
From www.numerade.com
SOLVEDThe solubility of zinc oxalate, ZnC2 O4, in 0.0150 M ammonia is Zinc Ion Ammonia Zinc +2 ion and dilute ammonia solution reaction. Because it is a stronger. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Reactions of the hexaaqua. Zinc Ion Ammonia.
From www.researchgate.net
Electrochemical performances of AC//2 M ZnSO4//V2O5 zincion hybrid Zinc Ion Ammonia Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Following tests are done. Zinc Ion Ammonia.
From www.sciencephoto.com
Zinc sulphate in ammonia solution Stock Image C016/9564 Science Zinc Ion Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Because it is a stronger. Zinc +2 ion and dilute ammonia solution reaction. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Burns readily in air to form white zno and combines with. Zinc Ion Ammonia.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. Zinc +2 ion and dilute ammonia solution reaction. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Polyatomic ions are. Zinc Ion Ammonia.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different. Zinc Ion Ammonia.
From www.youtube.com
How to Write the Net Ionic Equation for NaCl + Zn(NO3)2 = ZnCl2 + NaNO3 Zinc Ion Ammonia Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Because it is a stronger. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. As an example of the formation of complex ions, consider the. Zinc Ion Ammonia.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The Zinc Ion Ammonia Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn. Zinc Ion Ammonia.
From www.britannica.com
Zinc Properties, Uses, & Facts Britannica Zinc Ion Ammonia Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Zinc +2 ion and dilute ammonia solution reaction. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. As an example of the formation of complex ions, consider the addition. Zinc Ion Ammonia.
From slideplayer.com
Chapter 11 EDTA Titrations ppt download Zinc Ion Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2. Zinc Ion Ammonia.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Because it is a stronger. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with ammonia. Zinc Ion Ammonia.
From fr.dreamstime.com
Molécule De Cation D'ammonium Nh4 Et D'ammoniac Nh3. Formule Chimique Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Following. Zinc Ion Ammonia.
From www.researchgate.net
Crosslinking reaction between zinc ion and acidic functional monomer Zinc Ion Ammonia Zinc +2 ion and dilute ammonia solution reaction. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. When aqueous dilute ammonia solution is added slowly to the colourless zn. Zinc Ion Ammonia.
From byjus.com
A compound is heated with zinc dust and ammonium chloride followed by Zinc Ion Ammonia When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Following tests are done to identify zn 2+ ion and they. Zinc Ion Ammonia.
From www.dlt.ncssm.edu
TIGER NCSSM Distance Education and Extended Programs Zinc Ion Ammonia Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous. Zinc Ion Ammonia.
From pixels.com
Zinc Sulphate In Ammonia Solution Photograph by Martyn F. Chillmaid Zinc Ion Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Because it is a stronger. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Polyatomic ions are covalent units (molecules) where. Zinc Ion Ammonia.
From www.slideserve.com
PPT Complex Ions PowerPoint Presentation, free download ID2835720 Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Zinc +2 ion. Zinc Ion Ammonia.
From www.numerade.com
SOLVEDThe reaction of a drycell battery may be represented as follows Zinc Ion Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Because it is a stronger. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation. Zinc Ion Ammonia.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Zinc Ion Ammonia Because it is a stronger. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Burns readily in air to form white zno and combines with many nonmetals. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Following tests are done. Zinc Ion Ammonia.
From www.numerade.com
SOLVED Zinc metal reacts with concentrated nitric acid to give zinc Zinc Ion Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Because it is a stronger. Polyatomic ions are covalent units (molecules) where the total. Zinc Ion Ammonia.
From www.slideserve.com
PPT Common Ions by Charge PowerPoint Presentation, free download ID Zinc Ion Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Zinc +2 ion and dilute ammonia solution reaction. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions.. Zinc Ion Ammonia.
From www.numerade.com
SOLVEDZinc metal reacts with concentrated nitric acid to give zinc ion Zinc Ion Ammonia Burns readily in air to form white zno and combines with many nonmetals. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Zinc +2 ion and dilute ammonia solution reaction. Reactions of the hexaaqua ions with ammonia solution are. Zinc Ion Ammonia.
From www.researchgate.net
Schematics of the chemistry of the zincion battery. Zn²⁺ ions migrate Zinc Ion Ammonia Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. As an example of the formation of complex ions, consider the addition of. Zinc Ion Ammonia.
From www.researchgate.net
Impacts of ammonia loading on ZnO NPs toxicity to ammonia oxidation Zinc Ion Ammonia Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Zinc +2 ion and dilute ammonia solution reaction. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Following tests are done to identify zn 2+ ion and they. Zinc Ion Ammonia.
From exotrzyaw.blob.core.windows.net
Zinc Metal Ion at Myrtle Vanwormer blog Zinc Ion Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Zinc(ii) ion reacts with aqueous ammonia to precipitate. Zinc Ion Ammonia.
From namingcomplexions.blogspot.com
Complex Ions Zinc Ion Ammonia Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Because it is a stronger. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Burns readily in air to form white. Zinc Ion Ammonia.
From pubs.acs.org
Hydrolysis Mechanism of WaterSoluble Ammonium Polyphosphate Affected Zinc Ion Ammonia Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Zinc +2 ion and dilute ammonia solution reaction. Because it is a stronger. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). When aqueous dilute ammonia. Zinc Ion Ammonia.
From www.fishersci.com
Ammonium zinc sulfate hydrate, Thermo Scientific, Quantity 250g Zinc Ion Ammonia When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h. Zinc Ion Ammonia.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Ion Ammonia Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Reactions of the hexaaqua ions with ammonia solution are complicated by the. Zinc Ion Ammonia.
From www.researchgate.net
Reagents and conditions. (a) Ammonium chloride, zinc acetate, sodium Zinc Ion Ammonia Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Burns readily in air to form white zno and combines with many nonmetals. Because it is a stronger. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in. Zinc Ion Ammonia.
From www.mdpi.com
Metals Free FullText Hydrometallurgical Process for Zinc Recovery Zinc Ion Ammonia Zinc +2 ion and dilute ammonia solution reaction. Because it is a stronger. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Following tests are done to identify zn 2+ ion and they are explained in. Zinc Ion Ammonia.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Zinc Ion Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Burns readily in air to form white zno and combines with many nonmetals. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with ammonia zn(ii). Zinc Ion Ammonia.
From www.youtube.com
Test for Zinc ion with Aqueous Ammonia YouTube Zinc Ion Ammonia Because it is a stronger. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Following tests are done to identify zn 2+ ion and they are explained in detail in this tutorial. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc. Zinc Ion Ammonia.
From www.researchgate.net
Schematic illustration of the working principle of rechargeable Znion Zinc Ion Ammonia Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2 + ion {[cu(h 2 o) 6] 2+}. Reaction of zinc with. Zinc Ion Ammonia.
From www.youtube.com
How to Write the Net Ionic Equation for (NH4)3PO4 + Zn(NO3)2 = NH4NO3 Zinc Ion Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were introduced in section 2.6.4.2. Reactions of the hexaaqua ions with ammonia solution are complicated by the fact that the ammonia can have two quite different functions. Burns. Zinc Ion Ammonia.