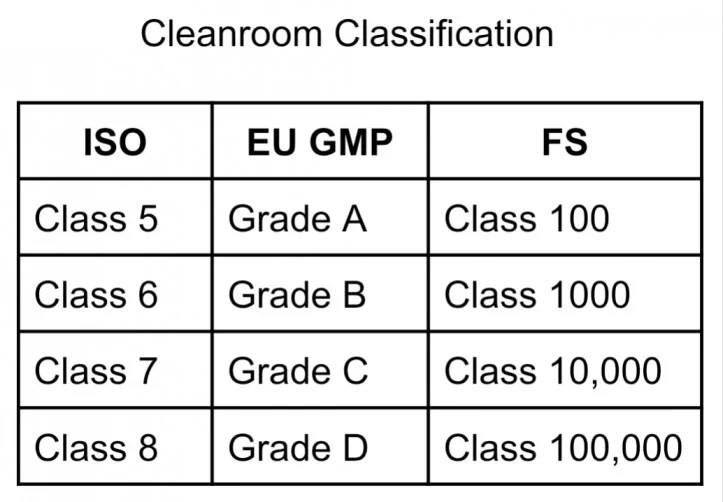
Who Pharmaceutical Clean Room Classification . The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Under the gmp requirements, the. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. Who good manufacturing practices for sterile pharmaceutical products. Following implementation of these who good. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. It determines how a room should be.
from www.pharmainform.com
Following implementation of these who good. Under the gmp requirements, the. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. It determines how a room should be. Who good manufacturing practices for sterile pharmaceutical products. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records.
Cleanroom Classification in Pharma Pharmainform
Who Pharmaceutical Clean Room Classification It determines how a room should be. Who good manufacturing practices for sterile pharmaceutical products. It determines how a room should be. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Under the gmp requirements, the. Following implementation of these who good. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and.
From mungfali.com
ISO Cleanroom Classification Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. It determines how a room should be. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. There should. Who Pharmaceutical Clean Room Classification.
From chemtech-us.com
What is a Clean Room? Pharmaceutical Cleanroom Classifications Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. Following implementation of these who good. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Who good manufacturing practices for. Who Pharmaceutical Clean Room Classification.
From brvalidations.blogspot.com
Pharmaceuticals Validation and stabilityTechnical Quality and Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Following implementation of these who good. It determines how a room should be. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed. Who Pharmaceutical Clean Room Classification.
From www.aroundlabnews.com
AROUND LAB NEWS / IT » Understanding Cleanroom Classifications Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Following implementation of these who good. It determines how a room should be. Who good manufacturing. Who Pharmaceutical Clean Room Classification.
From www.liberty-ind.com
The Design & Classifcations for Pharmaceutical Cleanrooms Liberty Who Pharmaceutical Clean Room Classification The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. Who good manufacturing practices for sterile pharmaceutical products. It determines how a room should be. Under the gmp requirements, the. Following implementation. Who Pharmaceutical Clean Room Classification.
From ansaripharmaeducation.blogspot.com
CLEAN ROOM CLASSIFICATION CLEAN AREA CLASSIFICATION IN MICROBIOLOGY Who Pharmaceutical Clean Room Classification Who good manufacturing practices for sterile pharmaceutical products. Following implementation of these who good. Under the gmp requirements, the. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. There should be adequate. Who Pharmaceutical Clean Room Classification.
From searose.com.au
The Clean Room Standards for Pharmaceuticals Searose Who Pharmaceutical Clean Room Classification An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Under the gmp requirements, the. It determines how a room should be. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. The gmp clean air grades. Who Pharmaceutical Clean Room Classification.
From www.ekeasgroup.com
Cleanroom standards Cleanroom classification Who Pharmaceutical Clean Room Classification For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and. Who Pharmaceutical Clean Room Classification.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Who Pharmaceutical Clean Room Classification It determines how a room should be. Following implementation of these who good. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room. Who Pharmaceutical Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Pharmaceutical Clean Room Classification The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. It determines how a room should be. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Under the gmp requirements, the. Who good. Who Pharmaceutical Clean Room Classification.
From www.slideserve.com
PPT What is a Pharmaceutical Cleanroom PowerPoint Presentation, free Who Pharmaceutical Clean Room Classification The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. It determines how a room should be. An area (or room or zone) with. Who Pharmaceutical Clean Room Classification.
From www.prefabcleanrooms.com
Hardwall Pharmaceutical Cleanroom Classification ISO 5 Clean Room Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Who good manufacturing practices for sterile pharmaceutical products. Following implementation of these who good. Under the gmp requirements, the. For initial classification, or classification following. Who Pharmaceutical Clean Room Classification.
From pharmagxp.com
Clean Room and Classification Summary of 4 Different Standards Who Pharmaceutical Clean Room Classification Who good manufacturing practices for sterile pharmaceutical products. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Following implementation of these who good. It determines how a room should be. There should. Who Pharmaceutical Clean Room Classification.
From www.golighthouse.com
Cleanroom Classifications Explained Lighthouse Worldwide Solutions Who Pharmaceutical Clean Room Classification Who good manufacturing practices for sterile pharmaceutical products. Following implementation of these who good. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. It determines how a room should be. An area (or room or zone) with defined environmental control of particulate and. Who Pharmaceutical Clean Room Classification.
From www.lmairtech.com
Cleanroom Classification & Design Guidelines LM AIR TECHNOLOGY Who Pharmaceutical Clean Room Classification The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. It determines how a room should be. Following implementation of these who good. Who good manufacturing practices for sterile pharmaceutical products. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. Under. Who Pharmaceutical Clean Room Classification.
From hibnhaat.blogspot.com
Iso Clean Room Classification An Overview of ISO 14644 Clean Room Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes. Who Pharmaceutical Clean Room Classification.
From www.presentationeze.com
Cleanroom Classification Information & current Best Who Pharmaceutical Clean Room Classification For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Under the gmp requirements, the. Following implementation of these who good. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. It determines how a room. Who Pharmaceutical Clean Room Classification.
From www.pharmaqualification.com
Pharmaceutical Clean Room Classification Most recent update of 14644 Who Pharmaceutical Clean Room Classification It determines how a room should be. Following implementation of these who good. Who good manufacturing practices for sterile pharmaceutical products. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered. Who Pharmaceutical Clean Room Classification.
From www.mecart-cleanrooms.com
Cleanroom Classifications (ISO 8, ISO 7, ISO 6, ISO 5) Who Pharmaceutical Clean Room Classification The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. It determines how a room should be. Following implementation of these who good. An. Who Pharmaceutical Clean Room Classification.
From www.cleanroom-industries.com
Pharmaceutical Cleanroom Components Who Pharmaceutical Clean Room Classification It determines how a room should be. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Following implementation of these who good. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Who good manufacturing practices for sterile pharmaceutical products. Under the. Who Pharmaceutical Clean Room Classification.
From us.ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Pharmaceutical Clean Room Classification An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Who good manufacturing practices for sterile pharmaceutical products. The gmp clean air grades and classifications define the. Who Pharmaceutical Clean Room Classification.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Who Pharmaceutical Clean Room Classification Following implementation of these who good. It determines how a room should be. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. Who good manufacturing practices for sterile pharmaceutical products. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. An. Who Pharmaceutical Clean Room Classification.
From corona.or.kr
Pharmaceutical Clean Room Classification Pdf corona.or.kr Who Pharmaceutical Clean Room Classification It determines how a room should be. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Who good manufacturing practices for sterile pharmaceutical products. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. There should. Who Pharmaceutical Clean Room Classification.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Who Pharmaceutical Clean Room Classification Following implementation of these who good. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. Who good manufacturing practices for sterile pharmaceutical products. It determines how a room should be. Under the gmp requirements, the. An area (or room or zone) with defined. Who Pharmaceutical Clean Room Classification.
From www.mecart-cleanrooms.com
Cleanroom Classifications (ISO 8, ISO 7, ISO 6, ISO 5) Who Pharmaceutical Clean Room Classification It determines how a room should be. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. There should be adequate suitable space for samples, reference organisms,. Who Pharmaceutical Clean Room Classification.
From operonstrategist.com
Clean Room Classification for Injection Molding and Assembly (A Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. It determines how a room should be. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Following implementation of these who good. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered. Who Pharmaceutical Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Pharmaceutical Clean Room Classification Who good manufacturing practices for sterile pharmaceutical products. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5. Who Pharmaceutical Clean Room Classification.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Who Pharmaceutical Clean Room Classification Who good manufacturing practices for sterile pharmaceutical products. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Under the gmp requirements, the. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. It determines how a room should be. Following implementation. Who Pharmaceutical Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Following implementation of these who good. Under the gmp requirements, the. For initial classification, or classification following significant change, it is recommended that both ≥0.5. Who Pharmaceutical Clean Room Classification.
From www.slideshare.net
Cleanroom, Classification, Design and Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. Following implementation of these who good. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Who good manufacturing practices for sterile pharmaceutical products. It determines how a. Who Pharmaceutical Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Pharmaceutical Clean Room Classification For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm particle sizes are considered for all grades of. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Following implementation of these who good. Who good manufacturing practices for sterile pharmaceutical products.. Who Pharmaceutical Clean Room Classification.
From pharmastate.academy
Cleanroom Classifications, Classes and ISO Standards Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who good manufacturing practices for sterile pharmaceutical products. Under the gmp requirements, the. An area (or room or zone) with defined environmental. Who Pharmaceutical Clean Room Classification.
From dycem.com
What Cleanroom Classification Does Your Industry Need? Dycem Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Under the gmp requirements, the.. Who Pharmaceutical Clean Room Classification.
From www.pharmainform.com
Cleanroom Classification in Pharma Pharmainform Who Pharmaceutical Clean Room Classification Under the gmp requirements, the. It determines how a room should be. There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. An area (or room or zone) with defined environmental control of particulate and microbial contamination, constructed and. Who good manufacturing practices for sterile pharmaceutical products. The gmp clean air grades. Who Pharmaceutical Clean Room Classification.
From joisijqwb.blob.core.windows.net
Clean Room Requirements For Pharmaceutical Manufacturing at Luis Allen blog Who Pharmaceutical Clean Room Classification There should be adequate suitable space for samples, reference organisms, media (if necessary, with cooling), testing and records. It determines how a room should be. The gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Following implementation of these who good. An area (or room or zone) with defined environmental. Who Pharmaceutical Clean Room Classification.