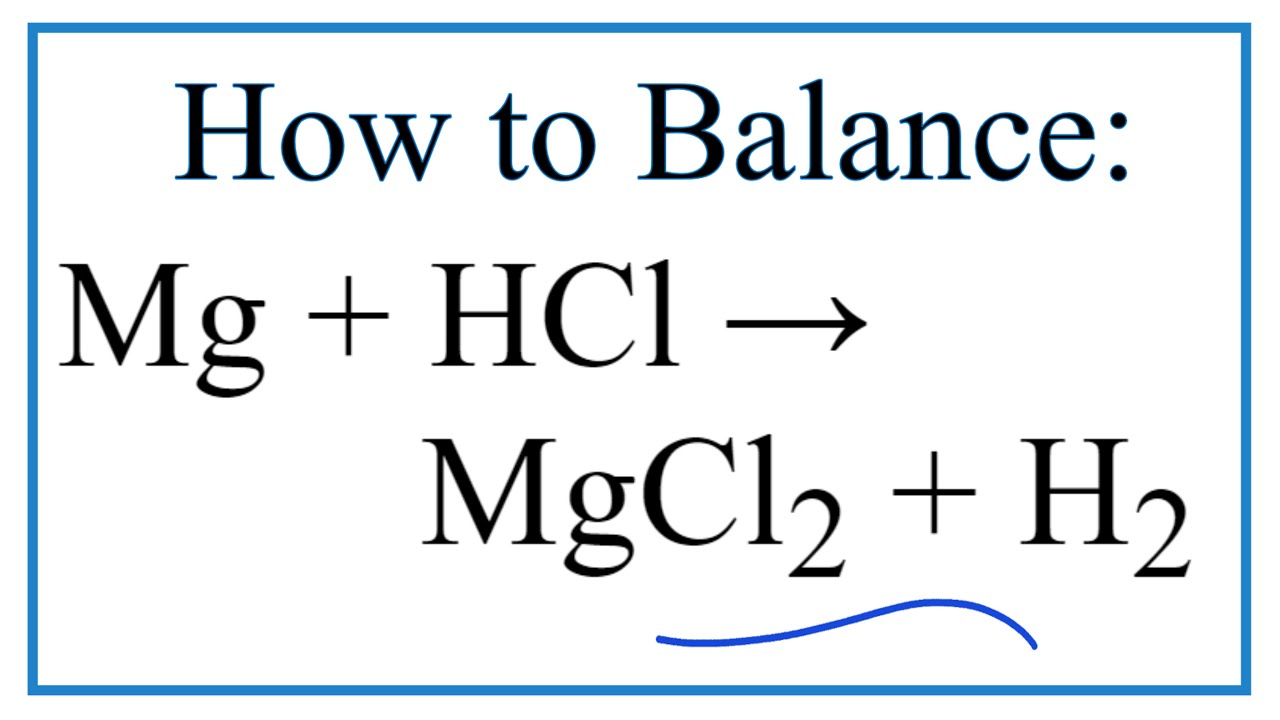
Magnesium Carbonate Hydrochloric Acid . The equation for the reaction is: Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The carbon dioxide forms a weak acid. Hydrochloric acid + zinc → zinc chloride +. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Acids react with metals to produce a salt and hydrogen. Enter an equation of an ionic chemical equation and press the balance button. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Acid + metal → salt + hydrogen. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The balanced equation will be calculated along with the.
from hadassah-blogmercer.blogspot.com
Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Hydrochloric acid + magnesium →. The balanced equation will be calculated along with the. The carbon dioxide forms a weak acid. Hydrochloric acid + zinc → zinc chloride +. Acids react with metals to produce a salt and hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Enter an equation of an ionic chemical equation and press the balance button. Magnesium + hydrochloric acid → magnesium chloride + hydrogen.
Ionic Equation of Magnesium and Hydrochloric Acid
Magnesium Carbonate Hydrochloric Acid Acid + metal → salt + hydrogen. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Enter an equation of an ionic chemical equation and press the balance button. The carbon dioxide forms a weak acid. Acids react with metals to produce a salt and hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Hydrochloric acid + zinc → zinc chloride +. Acid + metal → salt + hydrogen. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. The equation for the reaction is: The balanced equation will be calculated along with the. Acid + metal → salt + hydrogen. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between.
From omniscience.tech
Mastering Magnesium and Hydrochloric Acid Reactions Unraveling the Magnesium Carbonate Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Hydrochloric. Magnesium Carbonate Hydrochloric Acid.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Magnesium Carbonate Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Acid + metal → salt + hydrogen. The balanced equation will be calculated along with the. Enter an. Magnesium Carbonate Hydrochloric Acid.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Acid + metal → salt + hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acids will react with reactive metals, such as. Magnesium Carbonate Hydrochloric Acid.
From mavink.com
Magnesium And Hcl Reaction Magnesium Carbonate Hydrochloric Acid The carbon dioxide forms a weak acid. Hydrochloric acid + magnesium →. Hydrochloric acid + zinc → zinc chloride +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Mg + hcl = mgcl2 +. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
Magnesium Ribbon and Hydrochloric Acid Experiment YouTube Magnesium Carbonate Hydrochloric Acid Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Hydrochloric acid + magnesium →. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one. Magnesium Carbonate Hydrochloric Acid.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. The carbon dioxide forms a weak acid. The balanced equation will be calculated along with the. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg (s) + 2hcl (aq) → mgcl. Magnesium Carbonate Hydrochloric Acid.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Carbonate Hydrochloric Acid The carbon dioxide forms a weak acid. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Hydrochloric acid + zinc → zinc chloride +. Hydrochloric acid + magnesium →. Acids will react with reactive metals,. Magnesium Carbonate Hydrochloric Acid.
From www.slideshare.net
Chemical equations Magnesium Carbonate Hydrochloric Acid One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Acids react with metals to produce a salt and hydrogen. Enter an equation of an ionic chemical equation and press the balance button. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of. Magnesium Carbonate Hydrochloric Acid.
From oneclass.com
OneClass Magnesium carbonate reacts with hydrochloric acid to produce Magnesium Carbonate Hydrochloric Acid The equation for the reaction is: Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Acids react with metals to produce a salt and hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Acid + metal → salt + hydrogen. The carbon dioxide. Magnesium Carbonate Hydrochloric Acid.
From www.sciencephoto.com
Magnesium reacts with hydrochloric acid Stock Image C028/0732 Magnesium Carbonate Hydrochloric Acid The balanced equation will be calculated along with the. Hydrochloric acid + magnesium →. Acid + metal → salt + hydrogen. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Acid + metal → salt + hydrogen. One practical way to neutralize the basic ph is to bubble \. Magnesium Carbonate Hydrochloric Acid.
From www.sciencephoto.com
Magnesium reacts with hydrochloric acid Stock Image C028/0734 Magnesium Carbonate Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Hydrochloric acid + zinc → zinc chloride +. Magnesium +. Magnesium Carbonate Hydrochloric Acid.
From www.coursehero.com
[Solved] 5. when solid magnesium reacts with aqueous hydrochloric acid Magnesium Carbonate Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Enter an equation of an ionic chemical equation and press the balance button. Hydrochloric acid + zinc → zinc chloride +. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Acid + metal → salt + hydrogen. Hydrochloric acid +. Magnesium Carbonate Hydrochloric Acid.
From www.answersarena.com
[Solved] Write balanced chemical equation for the reaction Magnesium Carbonate Hydrochloric Acid Hydrochloric acid + magnesium →. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Hydrochloric acid + zinc → zinc chloride +. Enter an equation of an ionic chemical equation and press the balance button. Acids react with metals to produce a salt and hydrogen. Acid + metal → salt + hydrogen.. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for HCl + MgCO3 = MgCl2 + CO2 + H2O Magnesium Carbonate Hydrochloric Acid Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. The equation for the reaction is: Hydrochloric acid + zinc → zinc chloride +. Acids react with metals to produce a salt and hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and. Magnesium Carbonate Hydrochloric Acid.
From mungfali.com
Magnesium Carbonate Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid Enter an equation of an ionic chemical equation and press the balance button. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The equation for the reaction is: Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Hydrochloric acid + zinc → zinc chloride. Magnesium Carbonate Hydrochloric Acid.
From www.sciencephoto.com
Magnesium carbonate in hydrochloric acid Stock Image A500/0601 Magnesium Carbonate Hydrochloric Acid Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. The equation for the reaction is: The carbon dioxide forms a weak acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Enter an equation. Magnesium Carbonate Hydrochloric Acid.
From fineartamerica.com
Magnesium Carbonate In Hydrochloric Acid Photograph by Martyn F. Chillmaid Magnesium Carbonate Hydrochloric Acid The balanced equation will be calculated along with the. Acids react with metals to produce a salt and hydrogen. Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride +. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Mg + hcl = mgcl2 +. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
Magnesium & Hydrochloric Acid Reaction YouTube Magnesium Carbonate Hydrochloric Acid One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Enter an equation of an ionic chemical equation and press the balance button. The equation for the reaction is: Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
Magnesium reacting with Hydrochloric Acid YouTube Magnesium Carbonate Hydrochloric Acid One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Magnesium + hydrochloric acid → magnesium. Magnesium Carbonate Hydrochloric Acid.
From www.slideserve.com
PPT Reactions of Acids PowerPoint Presentation, free download ID Magnesium Carbonate Hydrochloric Acid Acid + metal → salt + hydrogen. Acids react with metals to produce a salt and hydrogen. The carbon dioxide forms a weak acid. Hydrochloric acid + magnesium →. Enter an equation of an ionic chemical equation and press the balance button. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg. Magnesium Carbonate Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Carbonate Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. Enter an equation of an ionic chemical equation and press the balance button. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Acid + metal → salt + hydrogen. The carbon dioxide forms. Magnesium Carbonate Hydrochloric Acid.
From koltenfersrubio.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. The balanced equation will be calculated along with the. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Hydrochloric acid + zinc → zinc chloride +. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium. Magnesium Carbonate Hydrochloric Acid.
From complianceportal.american.edu
💋 Rate of reaction of magnesium with hydrochloric acid. Rate of Magnesium Carbonate Hydrochloric Acid Magnesium + hydrochloric acid → magnesium chloride + hydrogen. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Enter an equation of an ionic chemical equation and press the balance button. Acid + metal → salt + hydrogen. The equation for the reaction is: The balanced equation will be calculated along with. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
Hydrochloric Acid and Magnesium Reaction YouTube Magnesium Carbonate Hydrochloric Acid Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate,. Magnesium Carbonate Hydrochloric Acid.
From koltenfersrubio.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Acid + metal → salt + hydrogen. The equation for the reaction is: The balanced equation will be calculated along with the. One practical way to neutralize the basic ph is to bubble \ (\ce. Magnesium Carbonate Hydrochloric Acid.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. The equation for the reaction is: Enter an equation of an ionic chemical equation and press the balance button. Hydrochloric acid + magnesium →. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. One. Magnesium Carbonate Hydrochloric Acid.
From www.numerade.com
SOLVEDA 6.53 g sample of a mixture of magnesium carbonate and calcium Magnesium Carbonate Hydrochloric Acid Acid + metal → salt + hydrogen. The equation for the reaction is: One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. The balanced equation will be. Magnesium Carbonate Hydrochloric Acid.
From segudanggambar798.blogspot.com
1. Solid Magnesium Oxide Is Reacted With Hydrochloric Acid Single Magnesium Carbonate Hydrochloric Acid One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The carbon dioxide forms a weak acid. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Hydrochloric acid. Magnesium Carbonate Hydrochloric Acid.
From www.youtube.com
Magnesium and Hydrochloric Acid Reaction YouTube Magnesium Carbonate Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Acid + metal → salt + hydrogen. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Mg + hcl = mgcl2 + h2 is a single displacement. Magnesium Carbonate Hydrochloric Acid.
From mungfali.com
Magnesium Hydrochloric Acid Reaction Magnesium Carbonate Hydrochloric Acid The equation for the reaction is: Mg (s) + 2hcl (aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When aqueous hydrochloric acid (hcl) is mixed with solid. Magnesium Carbonate Hydrochloric Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Magnesium Carbonate Hydrochloric Acid Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One practical way to neutralize. Magnesium Carbonate Hydrochloric Acid.
From www.yumpu.com
A Reaction Between Magnesium & Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Enter an equation of an ionic chemical equation and press the balance button. Hydrochloric acid + magnesium →. The equation for the reaction is: Acids react with metals to produce a salt and hydrogen. Acid + metal → salt +. Magnesium Carbonate Hydrochloric Acid.
From fineartamerica.com
Magnesium Reacting With Hydrochloric Acid Photograph by Martyn F Magnesium Carbonate Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. Enter an equation of an ionic chemical equation and press the balance button. Acids react with metals to produce a salt and hydrogen. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. When aqueous hydrochloric acid. Magnesium Carbonate Hydrochloric Acid.
From www.slideserve.com
PPT Energy Change During Chemical Reactions PowerPoint Presentation Magnesium Carbonate Hydrochloric Acid Acid + metal → salt + hydrogen. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Acid + metal → salt + hydrogen. The balanced equation will be calculated along with the. The equation for the reaction is: Acids will react with reactive metals, such as magnesium and zinc, to make a. Magnesium Carbonate Hydrochloric Acid.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate Hydrochloric Acid Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Acids react with metals to produce a salt and hydrogen. One practical way to neutralize the basic ph is to bubble \ (\ce {co_2}\) into the water. Magnesium + hydrochloric acid → magnesium chloride +. Magnesium Carbonate Hydrochloric Acid.