Does Vapor Pressure Increase With Surface Area . The vapor pressure is a measure of the pressure. Nothing happens to the vapour pressure as the surface area increases. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Vapour pressure is intensive property. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The vapour pressure of a liquid depends only on the temperature and its internal. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. Will the vapor pressure of the liquid increase or decrease? The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure.
from www.slideserve.com
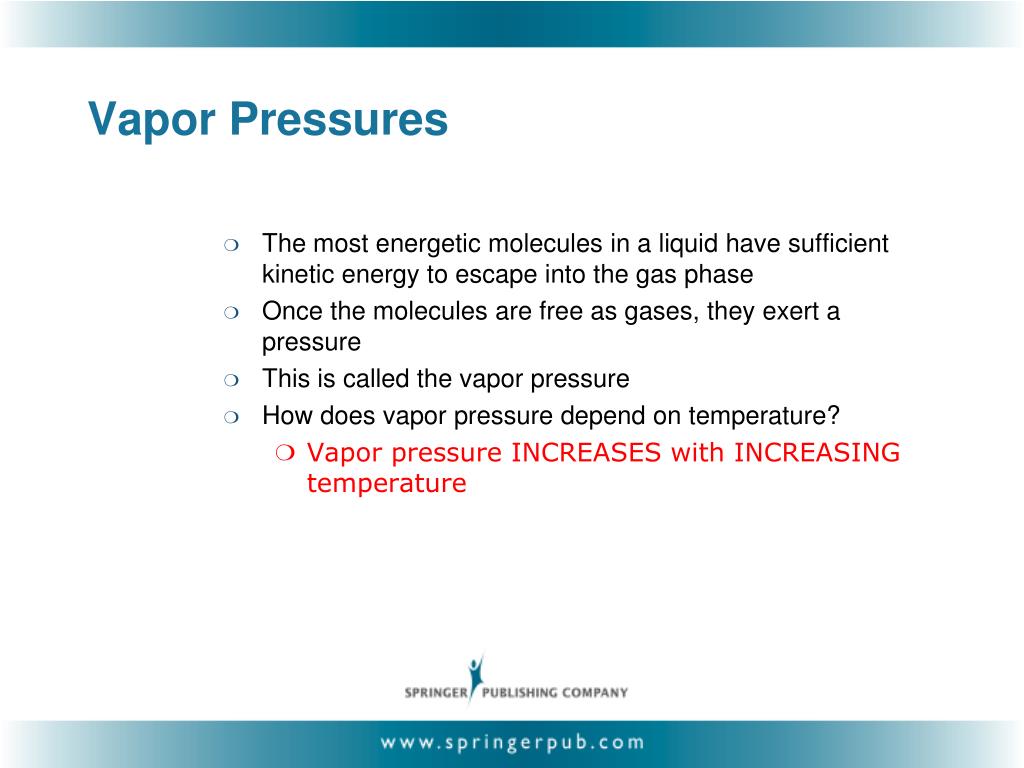
It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Vapour pressure is intensive property. The vapor pressure is a measure of the pressure. Nothing happens to the vapour pressure as the surface area increases. The vapour pressure of a liquid depends only on the temperature and its internal. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Will the vapor pressure of the liquid increase or decrease? Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container.
PPT Chapter 7 — States of Matter and Changes of State PowerPoint
Does Vapor Pressure Increase With Surface Area Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Will the vapor pressure of the liquid increase or decrease? Vapour pressure is intensive property. The vapor pressure is a measure of the pressure. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapour pressure of a liquid depends only on the temperature and its internal. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Nothing happens to the vapour pressure as the surface area increases.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Does Vapor Pressure Increase With Surface Area Vapour pressure is intensive property. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Will the vapor pressure of the liquid increase. Does Vapor Pressure Increase With Surface Area.
From chemyam.blogspot.com
COLLIGATIVE PROPERTIES (Relative lowering of vapour pressure) Does Vapor Pressure Increase With Surface Area The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Vapour pressure is intensive property. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Properties of Gases PowerPoint Presentation, free download ID Does Vapor Pressure Increase With Surface Area Nothing happens to the vapour pressure as the surface area increases. The vapor pressure is a measure of the pressure. Vapour pressure is intensive property. The vapour pressure of a liquid depends only on the temperature and its internal. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature. Does Vapor Pressure Increase With Surface Area.
From www.nuclear-power.com
Saturation Vapor Curve Nuclear Power Does Vapor Pressure Increase With Surface Area Vapour pressure is intensive property. The vapor pressure is a measure of the pressure. Nothing happens to the vapour pressure as the surface area increases. Will the vapor pressure of the liquid increase or decrease? Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The. Does Vapor Pressure Increase With Surface Area.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Does Vapor Pressure Increase With Surface Area The vapour pressure of a liquid depends only on the temperature and its internal. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the. Does Vapor Pressure Increase With Surface Area.
From www.iqsdirectory.com
Ultrasonic Cleaning What Is It? How Does It Work? Types Of Does Vapor Pressure Increase With Surface Area It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. The vapour pressure of a liquid depends only on the temperature and its internal. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Chapter 10Solutions PowerPoint Presentation, free download ID Does Vapor Pressure Increase With Surface Area Will the vapor pressure of the liquid increase or decrease? Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapour pressure of a liquid depends only on the temperature and its internal. Because they cannot escape the container, the vapor molecules above the. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Chapter 10Solutions PowerPoint Presentation, free download ID Does Vapor Pressure Increase With Surface Area Vapour pressure is intensive property. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapor pressure stays the same as the surface area increases because the rates of. Does Vapor Pressure Increase With Surface Area.
From slidetodoc.com
Aim How does temperature affect the vapor pressure Does Vapor Pressure Increase With Surface Area Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. The vapor pressure is a measure of the pressure. The pressure exerted by the gas in equilibrium with a solid or liquid. Does Vapor Pressure Increase With Surface Area.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Does Vapor Pressure Increase With Surface Area It depends for pure liquids (if we neglect minor secondary effects) only on temperature. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the. Does Vapor Pressure Increase With Surface Area.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Vapor Pressure Increase With Surface Area Vapour pressure is intensive property. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Because they cannot escape the container, the. Does Vapor Pressure Increase With Surface Area.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. It depends. Does Vapor Pressure Increase With Surface Area.
From www.youtube.com
Vapour Pressure And Cavitation Basic Concepts Fluid Properties Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapour pressure of a liquid depends only on the temperature and its internal. The pressure exerted by the gas in equilibrium with a solid or. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download Does Vapor Pressure Increase With Surface Area It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The vapor pressure is a measure of the pressure. Vapour pressure is intensive property.. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Does Vapor Pressure Increase With Surface Area Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapour pressure of a liquid depends only on the. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Surface Area Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The pressure exerted by the gas in equilibrium with a solid. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT AP Chapter 11 PowerPoint Presentation, free download ID2275798 Does Vapor Pressure Increase With Surface Area Nothing happens to the vapour pressure as the surface area increases. Vapour pressure is intensive property. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Surface Area Nothing happens to the vapour pressure as the surface area increases. The vapour pressure of a liquid depends only on the temperature and its internal. Vapour pressure is intensive property. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. Because they cannot escape the. Does Vapor Pressure Increase With Surface Area.
From socratic.org
Explain how each of the following affects the vapour pressure of a Does Vapor Pressure Increase With Surface Area Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature. Does Vapor Pressure Increase With Surface Area.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Vapor Pressure Increase With Surface Area Will the vapor pressure of the liquid increase or decrease? Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapour pressure of a liquid depends only on the temperature and its internal. It depends for pure liquids (if we neglect minor secondary effects). Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Surface Area It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Will the vapor pressure of the liquid increase or decrease? Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The vapor. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Liquids, Solids, and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Surface Area Nothing happens to the vapour pressure as the surface area increases. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance.. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. It depends. Does Vapor Pressure Increase With Surface Area.
From www.chemistrylibrary.org
Vapor Pressure Does Vapor Pressure Increase With Surface Area It depends for pure liquids (if we neglect minor secondary effects) only on temperature. Will the vapor pressure of the liquid increase or decrease? The vapour pressure of a liquid depends only on the temperature and its internal. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of. Does Vapor Pressure Increase With Surface Area.
From www.studocu.com
Vapor pressure and its relationship with intermolecular forces The Does Vapor Pressure Increase With Surface Area Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. Nothing happens to the vapour pressure as the surface area increases. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID3483746 Does Vapor Pressure Increase With Surface Area Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. Vapour pressure is intensive property. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Will the vapor pressure of the liquid increase. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Does Vapor Pressure Increase With Surface Area The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. It turns out that having a nonvolatile solute in the liquid. Does Vapor Pressure Increase With Surface Area.
From www.slideshare.net
Chapter 11 Properties of Solutions Does Vapor Pressure Increase With Surface Area Nothing happens to the vapour pressure as the surface area increases. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The vapour pressure of a liquid depends only on the temperature and its internal. It depends for pure liquids (if we neglect minor secondary effects) only. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Does Vapor Pressure Increase With Surface Area Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The vapor pressure is a measure of the pressure. It depends for pure liquids (if we neglect minor secondary effects) only on temperature. The pressure exerted by the gas in equilibrium with a solid or liquid. Does Vapor Pressure Increase With Surface Area.
From saylordotorg.github.io
Vapor Pressure Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapour pressure of a liquid depends only on the temperature and its internal. Vapor pressure does not depend on surface area because it is. Does Vapor Pressure Increase With Surface Area.
From www.youtube.com
Vapor Pressure and Boiling YouTube Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. The vapour. Does Vapor Pressure Increase With Surface Area.
From circuitdbhandbook.z13.web.core.windows.net
Vapor Pressure Diagram Does Vapor Pressure Increase With Surface Area Vapour pressure is intensive property. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. It depends for pure liquids (if we neglect. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Chapter 7 — States of Matter and Changes of State PowerPoint Does Vapor Pressure Increase With Surface Area The vapor pressure is a measure of the pressure. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The pressure exerted by the gas in equilibrium with a solid or liquid in a closed container at a given temperature is called the vapor pressure. Will. Does Vapor Pressure Increase With Surface Area.
From socratic.org
What is the relation between critical temperature and boiling point or Does Vapor Pressure Increase With Surface Area It depends for pure liquids (if we neglect minor secondary effects) only on temperature. Will the vapor pressure of the liquid increase or decrease? Vapour pressure is intensive property. Because they cannot escape the container, the vapor molecules above the surface of the liquid exert a pressure on the walls of the container. The pressure exerted by the gas in. Does Vapor Pressure Increase With Surface Area.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Does Vapor Pressure Increase With Surface Area The vapour pressure of a liquid depends only on the temperature and its internal. Nothing happens to the vapour pressure as the surface area increases. It turns out that having a nonvolatile solute in the liquid will decrease the vapor pressure of the liquid because the solutes will. Because they cannot escape the container, the vapor molecules above the surface. Does Vapor Pressure Increase With Surface Area.