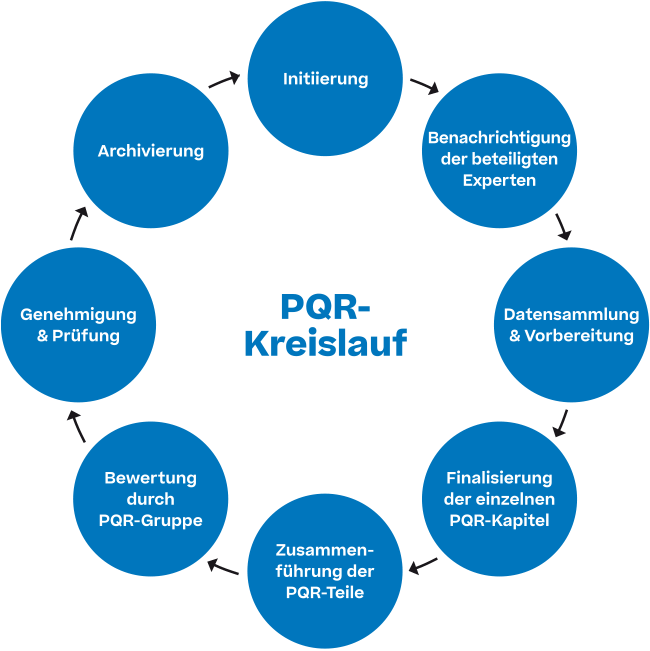
Product Quality Review Gmp . Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. Review timeframes can be appropriately adjusted based upon manufacturing and. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. A product quality review is generally expected annually. In the usa, the comparable document is called. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards.
from www.gmp-journal.de
(gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. In the usa, the comparable document is called. Review timeframes can be appropriately adjusted based upon manufacturing and. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. A product quality review is generally expected annually. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006.
Product Quality Review (PQR) Ein effektives Werkzeug zur
Product Quality Review Gmp The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. A product quality review is generally expected annually. In the usa, the comparable document is called. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Review timeframes can be appropriately adjusted based upon manufacturing and.
From www.phoenixmedical.com
Good Manufacturing Practice Product Quality Review Gmp All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. A product quality review is generally expected annually. In the usa, the comparable document is called. Product. Product Quality Review Gmp.
From www.gmp-journal.de
Product Quality Review (PQR) Ein effektives Werkzeug zur Product Quality Review Gmp All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. The gmps necessitate annual evaluation. Product Quality Review Gmp.
From allchem.asia
QUALITY CONTROL Product Quality Review Gmp (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Product quality reviews (pqrs) serve. Product Quality Review Gmp.
From www.attestationupdate.com
Major revision to Quality Control Standards on the horizon Product Quality Review Gmp This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing. Product Quality Review Gmp.
From www.vecteezy.com
Quality. Standard Quality Control Certification Assurance. Guarantee Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. In the usa, the comparable document is called. The. Product Quality Review Gmp.
From blogs.sas.com
A Holistic view of Product Quality SAS Voices Product Quality Review Gmp In the usa, the comparable document is called. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. All contracts in a “chain of contracts” setup are to be. Product Quality Review Gmp.
From xandrolab.com
GMPCertified What It Means for You Xandro Lab Product Quality Review Gmp A product quality review is generally expected annually. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. All contracts in a “chain of contracts” setup are to be reviewed as. Product Quality Review Gmp.
From www.indiamart.com
GMP Certification Service at Rs 100 in New Delhi ID 20484154088 Product Quality Review Gmp The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Review timeframes can be appropriately adjusted based upon manufacturing and. In the usa, the comparable document is. Product Quality Review Gmp.
From www.goldenhaven.com.ph
The Difference of Quality and Quantity Golden Haven Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. A product quality review is generally expected annually. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for. Product Quality Review Gmp.
From www.vectorstock.com
Good manufacturing practice gmp sign or stamp Vector Image Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards.. Product Quality Review Gmp.
From www.pdfprof.com
ein Qualitätsprodukt Product Quality Review Gmp Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. In the usa, the comparable document is called. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production. Product Quality Review Gmp.
From gmpinsiders.com
Product Quality Review PQR In GMP Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards.. Product Quality Review Gmp.
From www.pinterest.com
Annual Product Quality Review Software Trend analysis, Reviews Product Quality Review Gmp The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. In the usa, the comparable document is called. Review timeframes can be appropriately adjusted based upon manufacturing and. (gmp) for the manufacturing. Product Quality Review Gmp.
From www.optiproerp.com
What is Quality Management and why it is important? Product Quality Review Gmp A product quality review is generally expected annually. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. Review timeframes can be appropriately adjusted based upon manufacturing and. In the usa, the comparable document is called. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. The. Product Quality Review Gmp.
From qbdgroup.com
Annual Product Quality Review (APQR/PQR) in Pharma QbD Product Quality Review Gmp Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. In the usa, the comparable document is called. A product quality review is generally expected annually. Review timeframes can be appropriately adjusted based upon manufacturing and. All contracts in a “chain of contracts” setup are to be reviewed as part of the. Product Quality Review Gmp.
From www.5snews.com
Product Quality How can it be better Controlled? 5S News, Supplies Product Quality Review Gmp In the usa, the comparable document is called. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. The preparation of a product quality review is a requirement that was already. Product Quality Review Gmp.
From www.farmaceuticayounger.science
PQR = Product Quality Review Farmaceutica Younger Product Quality Review Gmp A product quality review is generally expected annually. Review timeframes can be appropriately adjusted based upon manufacturing and. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews (pqrs) serve as a cornerstone. Product Quality Review Gmp.
From www.webofpharma.com
Quality Risk Management in Pharmaceutical Plant Product Quality Review Gmp In the usa, the comparable document is called. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. The gmps necessitate annual evaluation of quality standards of a. Product Quality Review Gmp.
From www.slideshare.net
Product quality review Product Quality Review Gmp A product quality review is generally expected annually. In the usa, the comparable document is called. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. (gmp). Product Quality Review Gmp.
From q-support.be
Importance of Product Quality Reviews (PQRs) in Good Manufacturing Product Quality Review Gmp In the usa, the comparable document is called. Review timeframes can be appropriately adjusted based upon manufacturing and. A product quality review is generally expected annually. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating. Product Quality Review Gmp.
From www.pinterest.jp
The ten steps of annual product quality review Change management Product Quality Review Gmp A product quality review is generally expected annually. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production. Product Quality Review Gmp.
From www.vrogue.co
Gmp Compliance In Pharmaceutical Industry The Importa vrogue.co Product Quality Review Gmp This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. In the usa, the comparable document is called. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and. Product Quality Review Gmp.
From eniva.com
Eniva Health is GMP Certified Product Quality Review Gmp This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. Review timeframes can be appropriately adjusted based upon manufacturing and. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. Product quality reviews have become. Product Quality Review Gmp.
From www.gmtpharmainternational.com
How To Control The Quality Parameters In Pharmaceutical Manufacturing Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. A product quality review is generally. Product Quality Review Gmp.
From www.pngegg.com
Good manufacturing practice Best practice Quality, gmp, food, text png Product Quality Review Gmp Review timeframes can be appropriately adjusted based upon manufacturing and. In the usa, the comparable document is called. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. The gmps necessitate annual evaluation. Product Quality Review Gmp.
From www.rroij.com
Product Quality Reviews and Its Importance in Product Lifecycle Open Product Quality Review Gmp This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Review. Product Quality Review Gmp.
From www.vecteezy.com
GMP Good Manufacturing Practice 6 heading of infographic template with Product Quality Review Gmp (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. In the usa, the comparable document is called. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. All contracts in a “chain of contracts” setup are to be reviewed as. Product Quality Review Gmp.
From www.qmdocs.com
Quality Product Review / Annual Product Review Product Quality Review Gmp The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. The preparation of a product quality review is a requirement that was already included in. Product Quality Review Gmp.
From www.armorrite.com
Quality Assurance ArmorRite Security Doors & Windows Product Quality Review Gmp Review timeframes can be appropriately adjusted based upon manufacturing and. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards. In the usa, the comparable document is called. A product quality review is generally expected annually. All contracts in a “chain of contracts” setup are to be reviewed. Product Quality Review Gmp.
From kentuckybourbontrailmap.github.io
Gmp Indonesia Ensuring Quality And Safety In Pharmaceutical Production Product Quality Review Gmp Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. In the usa, the comparable document is called. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. The preparation of a product quality review is a requirement that was already included. Product Quality Review Gmp.
From gxpcellators.com
What's The Difference Between cGMP and GMP? Product Quality Review Gmp Review timeframes can be appropriately adjusted based upon manufacturing and. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every. Product Quality Review Gmp.
From www.slideserve.com
PPT 實施 PIC/S GMP 之藥廠應執行之相關作業 Product quality review system 與 持續性 Product Quality Review Gmp The preparation of a product quality review is a requirement that was already included in the eu gmp guideline in 2006. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. This systematic review confirms product quality and consistency and identifies areas for improvement, ensuring that every batch produced meets the highest standards.. Product Quality Review Gmp.
From thors.com
Advanced Product Quality Planning (APQP) Course Product Quality Review Gmp Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. (gmp) for the manufacturing of active pharmaceutical ingredients (apis) under an appropriate system for managing quality. Product quality reviews (pqrs) serve. Product Quality Review Gmp.
From www.pinterest.com
Download GMP. Good Manufacturing Practice infographic template for free Product Quality Review Gmp Product quality reviews have become an accepted part of gmp requirements internationally and can provide useful information and. Review timeframes can be appropriately adjusted based upon manufacturing and. A product quality review is generally expected annually. The gmps necessitate annual evaluation of quality standards of a drug product to determine the need for adjustments in drug product specifications, manufacturing. All. Product Quality Review Gmp.
From www.lifebiotic.com
gmp certified LifeBiotic Product Quality Review Gmp All contracts in a “chain of contracts” setup are to be reviewed as part of the product quality review (pqr) process. Product quality reviews (pqrs) serve as a cornerstone in gmp, comprehensively evaluating production and quality control processes. In the usa, the comparable document is called. The preparation of a product quality review is a requirement that was already included. Product Quality Review Gmp.