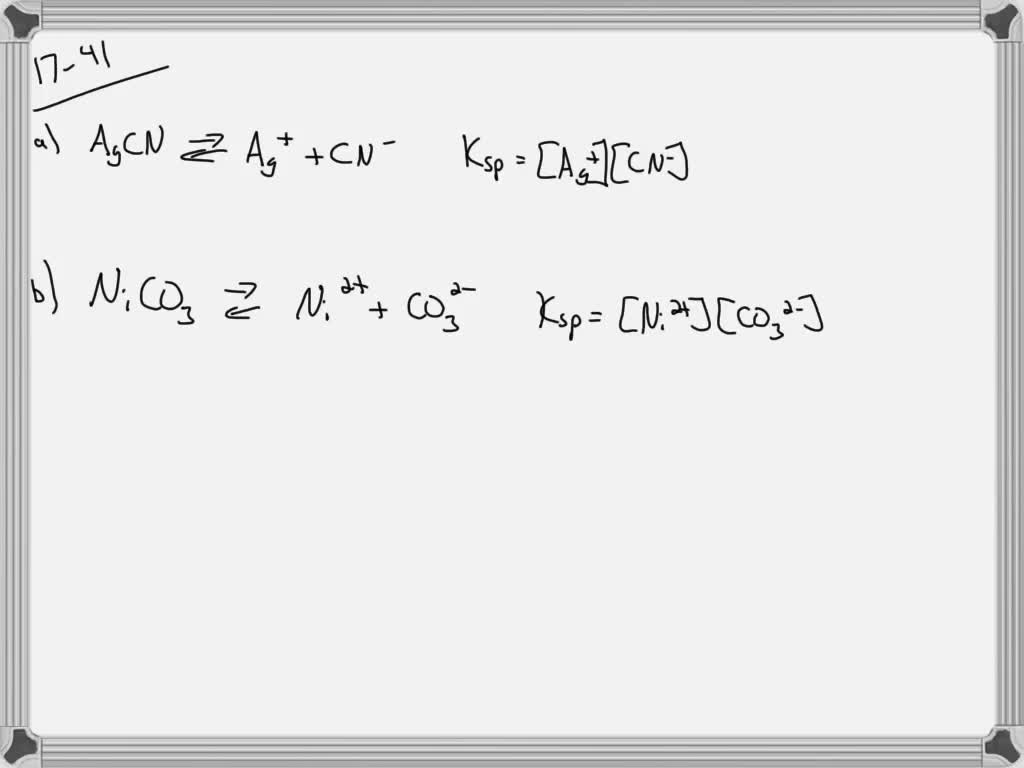Balanced Chemical Equation For Table Salt . the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. Na + cl 2 → 2nacl. balanced chemical equation of na + cl 2 reaction. Naoh + hcl → nacl + h₂o. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Here, sodium hydroxide reacts with hydrochloric acid to. sodium chloride = sodium + chlorine. Two moles of chlorine gas reacts with one mole of. if you want a dissociate equation it'll be written as follows: Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the balanced chemical equation for this process is: table salt is an ionic compound, which breaks into its component ions or dissociates in water. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than.
from www.numerade.com
sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. balanced chemical equation of na + cl 2 reaction. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. Here, sodium hydroxide reacts with hydrochloric acid to. sodium chloride = sodium + chlorine. Naoh + hcl → nacl + h₂o. table salt is an ionic compound, which breaks into its component ions or dissociates in water. if you want a dissociate equation it'll be written as follows: Na + cl 2 → 2nacl. Two moles of chlorine gas reacts with one mole of.
SOLVEDFor each of the following insoluble salts, (1) write a balanced
Balanced Chemical Equation For Table Salt if you want a dissociate equation it'll be written as follows: Here, sodium hydroxide reacts with hydrochloric acid to. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. the balanced chemical equation for this process is: the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. sodium chloride = sodium + chlorine. if you want a dissociate equation it'll be written as follows: Naoh + hcl → nacl + h₂o. table salt is an ionic compound, which breaks into its component ions or dissociates in water. Na + cl 2 → 2nacl. balanced chemical equation of na + cl 2 reaction. Two moles of chlorine gas reacts with one mole of. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than.
From www.youtube.com
Salt Equations Revision for ALevel Chemistry YouTube Balanced Chemical Equation For Table Salt balanced chemical equation of na + cl 2 reaction. if you want a dissociate equation it'll be written as follows: the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. the balanced chemical equation for this process is: Here, sodium hydroxide reacts with hydrochloric acid to. Two moles. Balanced Chemical Equation For Table Salt.
From www.numerade.com
SOLVED Give the balanced chemical equation for the dissolution of Balanced Chemical Equation For Table Salt the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. if you want a dissociate equation it'll be written as follows: table salt is an ionic compound, which breaks into its component ions or dissociates in water. Here, sodium hydroxide reacts with hydrochloric acid to. the balanced. Balanced Chemical Equation For Table Salt.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Balanced Chemical Equation For Table Salt the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. if you want a dissociate equation it'll be written as follows: Na + cl 2 → 2nacl. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. Here, sodium. Balanced Chemical Equation For Table Salt.
From www.animalia-life.club
Table Salt Chemical Structure Balanced Chemical Equation For Table Salt Na + cl 2 → 2nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. table salt is an ionic compound, which breaks into its component ions or dissociates in water. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. . Balanced Chemical Equation For Table Salt.
From exomdamui.blob.core.windows.net
Table Salt Atomic Formula at Pete Alvarez blog Balanced Chemical Equation For Table Salt if you want a dissociate equation it'll be written as follows: Na + cl 2 → 2nacl. Two moles of chlorine gas reacts with one mole of. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. table salt is an ionic compound, which breaks into its component ions. Balanced Chemical Equation For Table Salt.
From www.youtube.com
Given salt, find acid and base Chemistry Khan Academy YouTube Balanced Chemical Equation For Table Salt the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. Naoh + hcl → nacl + h₂o. sodium chloride = sodium + chlorine. Na + cl 2 → 2nacl. Two moles of chlorine gas reacts with one mole of. Here, sodium hydroxide reacts with hydrochloric acid to. Nacl =. Balanced Chemical Equation For Table Salt.
From ar.inspiredpencil.com
Chemical Formula For Salt Balanced Chemical Equation For Table Salt Two moles of chlorine gas reacts with one mole of. Naoh + hcl → nacl + h₂o. the balanced chemical equation for this process is: the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. balanced chemical equation of na + cl 2 reaction. Na + cl 2 →. Balanced Chemical Equation For Table Salt.
From riversfansite.blogspot.com
Table Salt Chemical Formula Sodium chloride , commonly known as salt Balanced Chemical Equation For Table Salt Here, sodium hydroxide reacts with hydrochloric acid to. balanced chemical equation of na + cl 2 reaction. Two moles of chlorine gas reacts with one mole of. table salt is an ionic compound, which breaks into its component ions or dissociates in water. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium. Balanced Chemical Equation For Table Salt.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Balanced Chemical Equation For Table Salt the balanced chemical equation for this process is: the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Naoh + hcl → nacl + h₂o. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. Here, sodium hydroxide reacts with. Balanced Chemical Equation For Table Salt.
From www.youtube.com
How to Write the Formula for Table Salt YouTube Balanced Chemical Equation For Table Salt Here, sodium hydroxide reacts with hydrochloric acid to. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Naoh + hcl → nacl + h₂o. Na + cl 2 → 2nacl. the balanced chemical equation for this process is: Nacl = na + cl is a decomposition reaction where one. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
Table Salt Chemical Compound Formula Elcho Table Balanced Chemical Equation For Table Salt the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Naoh + hcl → nacl + h₂o. sodium chloride = sodium + chlorine. balanced chemical equation of na + cl 2 reaction. Two moles of chlorine gas reacts with one mole of. table salt is an ionic compound,. Balanced Chemical Equation For Table Salt.
From www.alamy.com
Sodium chloride (table salt), chemical structure. Blue skeletal formula Balanced Chemical Equation For Table Salt Na + cl 2 → 2nacl. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. Here, sodium hydroxide reacts with hydrochloric acid to. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. table salt is an ionic compound, which breaks into. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
What Is The Chemical Formula For Table Salt Elcho Table Balanced Chemical Equation For Table Salt sodium chloride = sodium + chlorine. Naoh + hcl → nacl + h₂o. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. Na + cl 2 → 2nacl. the balanced chemical equation for this process is: Two moles of chlorine gas reacts with one mole of. . Balanced Chemical Equation For Table Salt.
From exooredui.blob.core.windows.net
Table Salt Is Acid Or Base at Martha Hanson blog Balanced Chemical Equation For Table Salt the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. balanced chemical equation of na + cl 2 reaction. Here, sodium hydroxide reacts with hydrochloric acid to. Naoh + hcl → nacl + h₂o. sodium chloride = sodium + chlorine. Two moles of chlorine gas reacts with one. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
Balanced Chemical Equation For Table Salt And Silver Nitrate Elcho Table Balanced Chemical Equation For Table Salt balanced chemical equation of na + cl 2 reaction. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. table salt is an ionic compound, which breaks into its component ions or dissociates in water. Two moles of chlorine gas reacts with one mole of. the simplest. Balanced Chemical Equation For Table Salt.
From www.youtube.com
salt hydrolysis and net ionic equations review YouTube Balanced Chemical Equation For Table Salt Na + cl 2 → 2nacl. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. the balanced chemical equation for this process is: Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. Two moles of chlorine gas reacts with one mole. Balanced Chemical Equation For Table Salt.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Balanced Chemical Equation For Table Salt Naoh + hcl → nacl + h₂o. sodium chloride = sodium + chlorine. the balanced chemical equation for this process is: if you want a dissociate equation it'll be written as follows: balanced chemical equation of na + cl 2 reaction. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination. Balanced Chemical Equation For Table Salt.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Balanced Chemical Equation For Table Salt Here, sodium hydroxide reacts with hydrochloric acid to. table salt is an ionic compound, which breaks into its component ions or dissociates in water. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and. Balanced Chemical Equation For Table Salt.
From learningzoneteiar09.z14.web.core.windows.net
Grade 10 Chemistry Balancing Equations Balanced Chemical Equation For Table Salt sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. Na + cl 2 → 2nacl. table salt is an ionic compound, which breaks into its component ions or dissociates in water. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
What Is The Chemical Equation For Table Salt Elcho Table Balanced Chemical Equation For Table Salt Naoh + hcl → nacl + h₂o. the balanced chemical equation for this process is: Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. Here, sodium hydroxide reacts with hydrochloric acid to. if you want a dissociate equation it'll be written as follows: table salt is an ionic compound, which. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Balanced Chemical Equation For Table Salt Two moles of chlorine gas reacts with one mole of. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. table salt is an ionic compound, which breaks into its component ions or dissociates in water. sodium chloride = sodium + chlorine. balanced chemical equation of na. Balanced Chemical Equation For Table Salt.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Balanced Chemical Equation For Table Salt Naoh + hcl → nacl + h₂o. table salt is an ionic compound, which breaks into its component ions or dissociates in water. the balanced chemical equation for this process is: Na + cl 2 → 2nacl. balanced chemical equation of na + cl 2 reaction. the synthesis reaction that produces table salt, or sodium chloride. Balanced Chemical Equation For Table Salt.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Balanced Chemical Equation For Table Salt sodium chloride = sodium + chlorine. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Two moles of chlorine gas reacts with one mole of. Na + cl 2 → 2nacl. if. Balanced Chemical Equation For Table Salt.
From www.nagwa.com
Question Video Identifying the Balanced Chemical Equation for the Balanced Chemical Equation For Table Salt Here, sodium hydroxide reacts with hydrochloric acid to. balanced chemical equation of na + cl 2 reaction. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. the balanced chemical equation for this process is: if you want a dissociate equation it'll be written as follows: . Balanced Chemical Equation For Table Salt.
From www.numerade.com
SOLVEDFor each of the following insoluble salts, (1) write a balanced Balanced Chemical Equation For Table Salt if you want a dissociate equation it'll be written as follows: Two moles of chlorine gas reacts with one mole of. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the balanced chemical equation for this process is: balanced chemical equation of na + cl 2 reaction. Here, sodium hydroxide. Balanced Chemical Equation For Table Salt.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Balanced Chemical Equation For Table Salt Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Here, sodium hydroxide reacts with hydrochloric acid to. Two moles of chlorine gas reacts with one mole of. the balanced chemical equation for this. Balanced Chemical Equation For Table Salt.
From www.alamy.com
an equation (chemistry) showing dissoatiation of table salt in water Balanced Chemical Equation For Table Salt the balanced chemical equation for this process is: Na + cl 2 → 2nacl. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. table salt is an ionic compound, which breaks into. Balanced Chemical Equation For Table Salt.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Balanced Chemical Equation For Table Salt Naoh + hcl → nacl + h₂o. sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. if you want a dissociate equation it'll be written as follows: the balanced chemical equation for this process is: table salt is an ionic compound, which breaks into its component. Balanced Chemical Equation For Table Salt.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Balanced Chemical Equation For Table Salt sodium chloride = sodium + chlorine. Here, sodium hydroxide reacts with hydrochloric acid to. Naoh + hcl → nacl + h₂o. the balanced chemical equation for this process is: Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. balanced chemical equation of na + cl 2 reaction. table salt. Balanced Chemical Equation For Table Salt.
From www.youtube.com
Balancing Chemical Equations 2 YouTube Balanced Chemical Equation For Table Salt table salt is an ionic compound, which breaks into its component ions or dissociates in water. Here, sodium hydroxide reacts with hydrochloric acid to. Two moles of chlorine gas reacts with one mole of. balanced chemical equation of na + cl 2 reaction. the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination. Balanced Chemical Equation For Table Salt.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Balanced Chemical Equation For Table Salt the synthesis reaction that produces table salt, or sodium chloride (nacl), involves the combination of sodium (na) and chlorine. table salt is an ionic compound, which breaks into its component ions or dissociates in water. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. sugar has the. Balanced Chemical Equation For Table Salt.
From thewritingparent.blogspot.com
Table Salt Chemical Formula F Wall Decoration Balanced Chemical Equation For Table Salt Na + cl 2 → 2nacl. if you want a dissociate equation it'll be written as follows: sodium chloride = sodium + chlorine. Naoh + hcl → nacl + h₂o. the balanced chemical equation for this process is: Here, sodium hydroxide reacts with hydrochloric acid to. the synthesis reaction that produces table salt, or sodium chloride. Balanced Chemical Equation For Table Salt.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Balanced Chemical Equation For Table Salt table salt is an ionic compound, which breaks into its component ions or dissociates in water. Here, sodium hydroxide reacts with hydrochloric acid to. balanced chemical equation of na + cl 2 reaction. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the simplest methods, where you examine and modify. Balanced Chemical Equation For Table Salt.
From elchoroukhost.net
What Is The Chemical Formula For Table Salt Elcho Table Balanced Chemical Equation For Table Salt sugar has the chemical formulate c12h22o11 c 12 h 22 o 11 and is constructed from different elements than. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. the synthesis reaction that. Balanced Chemical Equation For Table Salt.
From www.pinterest.co.uk
BALANCING CHEMICAL EQUATIONS LESSON PLAN A COMPLETE SCIENCE LESSON Balanced Chemical Equation For Table Salt the balanced chemical equation for this process is: sodium chloride = sodium + chlorine. the simplest methods, where you examine and modify coefficients in some systematic order, is generally called “balancing by. Nacl = na + cl is a decomposition reaction where one mole of aqueous sodium chloride. sugar has the chemical formulate c12h22o11 c 12. Balanced Chemical Equation For Table Salt.