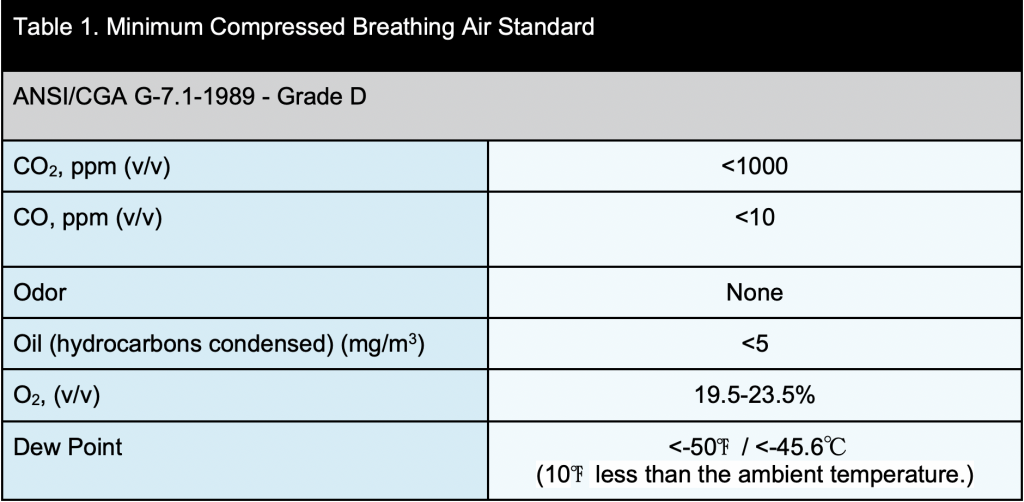
Gmp Compressed Air Requirements . For compressed air applications outside the clean room, the quality requirements are less clearly defined. Chad larrabee, global product management leader of ingersoll rand and gpg: All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. A reference point for the selection of. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of.
from www.airchecklab.com
This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Chad larrabee, global product management leader of ingersoll rand and gpg: A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are less clearly defined.
Meet OSHA Respiratory Protection Standards with Compressed Air Testing
Gmp Compressed Air Requirements To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. A reference point for the selection of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. Chad larrabee, global product management leader of ingersoll rand and gpg:
From schillingengineering.de
Cleanroom classes according to ISO 146441 and GMP Gmp Compressed Air Requirements A reference point for the selection of. Chad larrabee, global product management leader of ingersoll rand and gpg: For compressed air applications outside the clean room, the quality requirements are less clearly defined. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. All utilities that could affect. Gmp Compressed Air Requirements.
From pharmastate.academy
EudraLex Volume 4Good manufacturing Practice (GMP) guidelines Gmp Compressed Air Requirements All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. Chad larrabee, global product management leader of ingersoll rand and gpg: This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. The fda and. Gmp Compressed Air Requirements.
From airenergyuk.com
Compressed Breathing Air Quality Testing Air Energy Gmp Compressed Air Requirements For compressed air applications outside the clean room, the quality requirements are less clearly defined. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. A reference point for the selection of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air. Gmp Compressed Air Requirements.
From www.scribd.com
Requirements For Compressed Air in The Pharmaceutical Industry Gmp Compressed Air Requirements National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. Chad larrabee, global product management leader of ingersoll rand and gpg: All. Gmp Compressed Air Requirements.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Gmp Compressed Air Requirements To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. A reference point for the selection of. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. All utilities that could affect product quality. Gmp Compressed Air Requirements.
From www.phchd.com
ISO Certification and GMP Cleanroom Standards PHCbi Gmp Compressed Air Requirements For compressed air applications outside the clean room, the quality requirements are less clearly defined. Chad larrabee, global product management leader of ingersoll rand and gpg: This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. To adopt the specification in the pharmacopoeia. Gmp Compressed Air Requirements.
From www.slideserve.com
PPT GMP Programs PowerPoint Presentation, free download ID4741460 Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation,. Gmp Compressed Air Requirements.
From www.gmp-publishing.com
Air Handling Systems GMPVerlag Peither AG Gmp Compressed Air Requirements National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. The fda and eu gmps also mention that compressed gases should. Gmp Compressed Air Requirements.
From compressedairservices.com
Compressed Air Requirements By Compressed Air Services Gmp Compressed Air Requirements All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. For compressed air applications outside the clean room, the quality requirements are less clearly defined. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the.. Gmp Compressed Air Requirements.
From pharmaceutical.report
Pharmaceutical Compressed Air Quality GMP Standards And Requirements Gmp Compressed Air Requirements A reference point for the selection of. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. Chad larrabee, global product management leader of ingersoll rand and gpg: All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. National standards bodies. Gmp Compressed Air Requirements.
From www.airchecklab.com
Meet OSHA Respiratory Protection Standards with Compressed Air Testing Gmp Compressed Air Requirements Chad larrabee, global product management leader of ingersoll rand and gpg: To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation,. Gmp Compressed Air Requirements.
From www.researchandmarkets.com
Pharmaceutical Compressed Air Quality GMP Standards and Requirements Gmp Compressed Air Requirements National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. Chad larrabee, global product management leader of ingersoll rand and gpg: The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. This. Gmp Compressed Air Requirements.
From www.youtube.com
Single Use Guidelines according to EU GMP Annex 1, rev 12 2020 YouTube Gmp Compressed Air Requirements All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. Chad larrabee, global product management leader of ingersoll rand and gpg: National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. For compressed air applications outside the clean room,. Gmp Compressed Air Requirements.
From www.airchecklab.com
How to Designate ISO 85731 Purity Classes Trace Analytics, the Gmp Compressed Air Requirements This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. Chad larrabee, global product management leader of ingersoll rand and gpg: National standards bodies. Gmp Compressed Air Requirements.
From xuantong.com.bd
PCW and CDA System Xuan Tong BD Co., Ltd Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. For compressed air applications outside the clean room, the quality requirements are less clearly defined. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be.. Gmp Compressed Air Requirements.
From id.scribd.com
PDF Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. A reference point for the selection of. National standards bodies have guidance documents for. Gmp Compressed Air Requirements.
From www.golighthouse.com
GMP Annex 1 2022 Update Breakdown Part 1 Lighthouse Worldwide Solutions Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. A reference point for the selection of. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and.. Gmp Compressed Air Requirements.
From www.youtube.com
Pharmaceutical compressed air quality GMP standards and requirements Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. A reference point for the selection of. This guide covers water systems, pure steam,. Gmp Compressed Air Requirements.
From www.industrialair.co.nz
Compressed air quality standards Gmp Compressed Air Requirements For compressed air applications outside the clean room, the quality requirements are less clearly defined. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. Chad larrabee, global product management leader of ingersoll rand and gpg: A reference point for the selection of. This. Gmp Compressed Air Requirements.
From www.scribd.com
URS For GMP Facility PDF Specification (Technical Standard) Gmp Compressed Air Requirements All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are. Gmp Compressed Air Requirements.
From www.ifsqn.com
Compressed Air How Clean is Yours? International Food Safety and Gmp Compressed Air Requirements For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for. Gmp Compressed Air Requirements.
From ppt-online.org
Qualification and maintenance on GMP Air Handling Systems презентация Gmp Compressed Air Requirements Chad larrabee, global product management leader of ingersoll rand and gpg: The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. A reference point for the selection of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air. Gmp Compressed Air Requirements.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICE FOR BIOPROCESS ENGINEERING (ERT 421 Gmp Compressed Air Requirements To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. All utilities that. Gmp Compressed Air Requirements.
From nationalsafety.wordpress.com
Top 10 Compressed Air Safety Guidelines (Infographic) Nationalsafety Gmp Compressed Air Requirements To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. Chad larrabee, global product management leader of ingersoll rand and gpg: This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. For compressed. Gmp Compressed Air Requirements.
From www.airchecklab.com
Compressed Air System Risk Assessment Do I Need to Test? Trace Gmp Compressed Air Requirements This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating,. Gmp Compressed Air Requirements.
From www.safewellsolutions.co.uk
Compressed Air Quality Testing & Validation in accordance with ISO 8573 Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. Chad larrabee, global product management leader of ingersoll rand and gpg: This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining. Gmp Compressed Air Requirements.
From dokumen.tips
(PDF) Grade D Compressed Breathing Air Requirements … · air described Gmp Compressed Air Requirements This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. For compressed air applications outside the clean room, the quality requirements are less clearly defined. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should. Gmp Compressed Air Requirements.
From www.youtube.com
ISPE Good Practice Guide Critical Utilities GMP Compliance YouTube Gmp Compressed Air Requirements This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. A reference point for the selection of. For compressed air applications outside the clean room, the quality requirements are less clearly defined. National standards bodies have guidance documents for compressed air sampling, and. Gmp Compressed Air Requirements.
From solutionpharmacy.in
GMP Requirements Solution Parmacy Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. Chad larrabee, global product management leader of ingersoll rand and gpg: A reference point. Gmp Compressed Air Requirements.
From products.empire-airblast.com
Item 114013, EconoFinish® Blast On Empire Abrasive Gmp Compressed Air Requirements National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. A reference point for the selection of. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. Chad larrabee, global product management leader of ingersoll rand and gpg:. Gmp Compressed Air Requirements.
From www.dvelop-ls.de
EU GMP Guidelines Chapter 4 Learn more here in the glossary Gmp Compressed Air Requirements For compressed air applications outside the clean room, the quality requirements are less clearly defined. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the.. Gmp Compressed Air Requirements.
From pharmaceutical.report
Pharmaceutical Compressed Air Quality GMP Standards And Requirements Gmp Compressed Air Requirements The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. To adopt the specification in the pharmacopoeia unseen in the specification for a compressed air system for the production of. All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation,. Gmp Compressed Air Requirements.
From www.industrialoutpost.com
Compressed Air Requirements Chart Industrial Outpost The Official Gmp Compressed Air Requirements This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. Chad larrabee, global product management leader of ingersoll rand and gpg: All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. A reference point. Gmp Compressed Air Requirements.
From es.scribd.com
A Guide To The Work in Compressed Air Regulations 1996 Gmp Compressed Air Requirements All utilities that could affect product quality (e.g., steam, gas, compressed air, heating, ventilation, and air conditioning) should be. The fda and eu gmps also mention that compressed gases should have similar or better physical and microbiological quality than the cleanroom air located in the. A reference point for the selection of. For compressed air applications outside the clean room,. Gmp Compressed Air Requirements.
From www.youtube.com
An Introduction to EU GMP ( European Union Good Manufacturing Practices Gmp Compressed Air Requirements Chad larrabee, global product management leader of ingersoll rand and gpg: National standards bodies have guidance documents for compressed air sampling, and reference is made within fda and eu gmps, the general approach. For compressed air applications outside the clean room, the quality requirements are less clearly defined. A reference point for the selection of. This guide covers water systems,. Gmp Compressed Air Requirements.