What Determines Heat Capacity . Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. It is measured in joules per. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial role in understanding. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The specific heat of a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. In equation form, heat capacity c is c = m c,. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,.
from studylib.net
Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). In equation form, heat capacity c is c = m c,. The specific heat of a. It is usually expressed as calories per degree in. It is measured in joules per. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount.
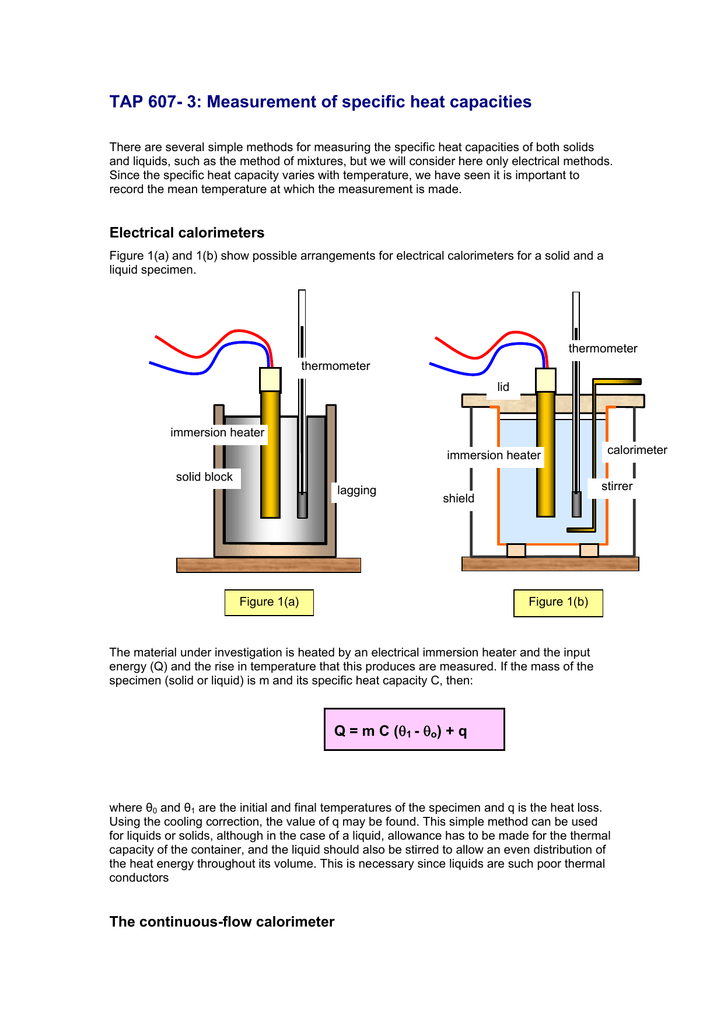
TAP 607 3 Measurement of specific heat capacities
What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. The specific heat of a. It is measured in joules per. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. In equation form, heat capacity c is c = m c,. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\).
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube What Determines Heat Capacity It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a. What Determines Heat Capacity.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube What Determines Heat Capacity Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The specific heat of a. The heat capacity (c) of a body. What Determines Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download What Determines Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It is usually expressed as calories per degree in. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when. What Determines Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Determines Heat Capacity Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. It is usually expressed as. What Determines Heat Capacity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Determines Heat Capacity The specific heat of a. It is measured in joules per. It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The heat capacity (c) of a body of matter is the. What Determines Heat Capacity.
From obropolox.blogspot.com
40 specific heat capacity worksheet answers Worksheet Resource What Determines Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The specific heat of a. It is measured in joules. What Determines Heat Capacity.
From www.slideshare.net
Heat Capacity What Determines Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It is usually expressed as calories per degree in. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The. What Determines Heat Capacity.
From www.slideserve.com
PPT Heat capacity PowerPoint Presentation, free download ID1419022 What Determines Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It is usually expressed as calories. What Determines Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Determines Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It is measured in joules per. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or. What Determines Heat Capacity.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Determines Heat Capacity In equation form, heat capacity c is c = m c,. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It. What Determines Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind What Determines Heat Capacity Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It is measured in joules per. Heat capacity is the amount of heat necessary to change the temperature of. What Determines Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Determines Heat Capacity It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by. What Determines Heat Capacity.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Determines Heat Capacity The specific heat of a. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Heat capacity is the amount of heat required to raise the temperature of an. What Determines Heat Capacity.
From studylib.net
Heat Capacity of Metals PreLab What Determines Heat Capacity It is measured in joules per. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of. What Determines Heat Capacity.
From www.markedbyteachers.com
Finding a material's specific heat capacity GCSE Science Marked by What Determines Heat Capacity The specific heat of a. It is usually expressed as calories per degree in. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It is measured in joules per. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00. What Determines Heat Capacity.
From studylib.net
1.3 Specific Heat Capacity What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree. What Determines Heat Capacity.
From haipernews.com
How To Determine Heat Capacity Using Dsc Haiper What Determines Heat Capacity In equation form, heat capacity c is c = m c,. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of. What Determines Heat Capacity.
From www.toppr.com
Describe a method to determine the specific heat capacity of a solid What Determines Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The specific heat of a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree. What Determines Heat Capacity.
From www.slideserve.com
PPT Specific Heat vs. Heat capacity PowerPoint Presentation, free What Determines Heat Capacity The specific heat of a. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one. What Determines Heat Capacity.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards What Determines Heat Capacity It plays a crucial role in understanding. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. It is measured in joules per. The heat. What Determines Heat Capacity.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Determines Heat Capacity It is measured in joules per. In equation form, heat capacity c is c = m c,. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. What Determines Heat Capacity.
From studylib.net
Heat Equation What Determines Heat Capacity It plays a crucial role in understanding. It is usually expressed as calories per degree in. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity. What Determines Heat Capacity.
From www.slideserve.com
PPT Heat Capacity and Specific Heat Capacity PowerPoint Presentation What Determines Heat Capacity It is measured in joules per. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. What Determines Heat Capacity.
From www.youtube.com
Specific heat capacity YouTube What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Specific heat is defined as the amount of heat required to raise the temperature. What Determines Heat Capacity.
From studylib.net
TAP 607 3 Measurement of specific heat capacities What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity, ratio of heat absorbed by a material to the temperature change. In equation form, heat capacity c is c = m c,. The heat capacity (c) of a body of matter is the quantity of. What Determines Heat Capacity.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Determines Heat Capacity In equation form, heat capacity c is c = m c,. It is usually expressed as calories per degree in. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Specific heat is defined as the amount of heat required to raise the temperature of a unit. What Determines Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Determines Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is measured in joules per. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change. What Determines Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube What Determines Heat Capacity Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). The specific heat of a. In equation form, heat capacity c is c = m c,. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The. What Determines Heat Capacity.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical What Determines Heat Capacity The specific heat of a. It is usually expressed as calories per degree in. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one. What Determines Heat Capacity.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Determines Heat Capacity The specific heat of a. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It plays a crucial role in understanding. Heat capacity is the measurable physical quantity that. What Determines Heat Capacity.
From www.tutorix.com
To determine specific heat capacity of given solid by method of mixtures What Determines Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. What Determines Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. It plays a crucial role in understanding. In equation form, heat capacity c is c = m c,. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of. What Determines Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Determines Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is measured in joules per. Heat capacity, ratio of heat absorbed. What Determines Heat Capacity.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Determines Heat Capacity The specific heat of a. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. It is usually expressed as calories per degree in. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It plays a crucial role. What Determines Heat Capacity.
From www.youtube.com
Specific heat capacity of solid by the method of mixture YouTube What Determines Heat Capacity In equation form, heat capacity c is c = m c,. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a. Heat capacity is the amount of heat required to raise the temperature of an object by \(1^\text{o} \text{c}\). It plays a crucial role in understanding. Specific. What Determines Heat Capacity.