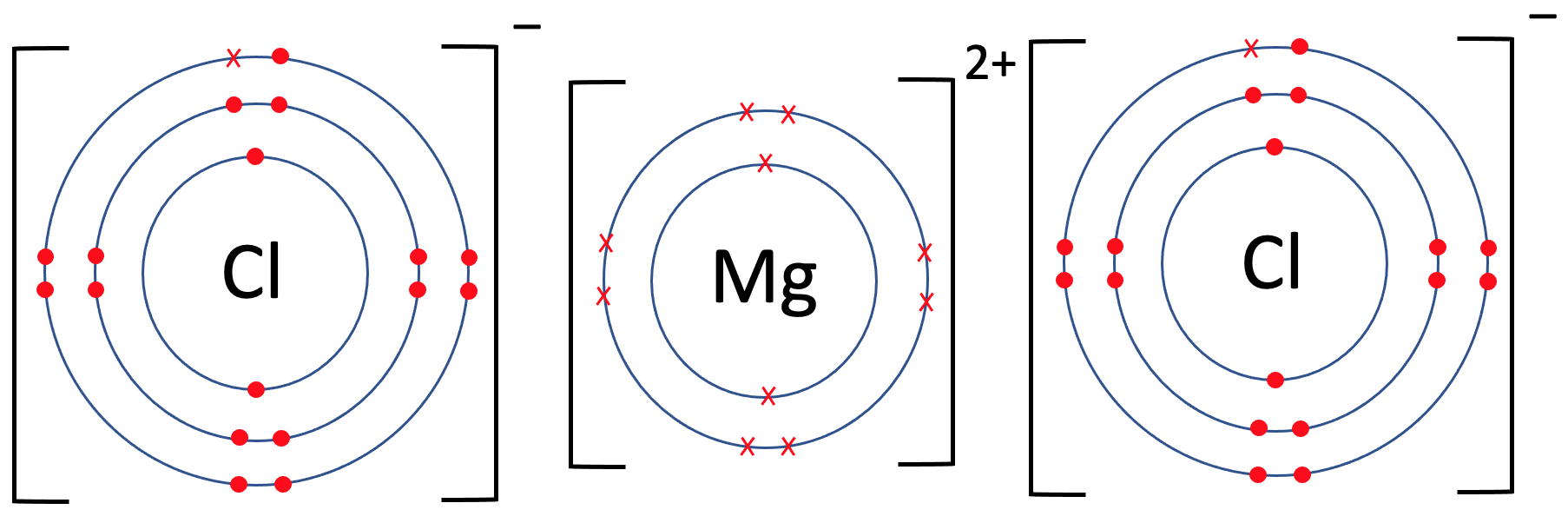
Chlorine Is Ionic . The 2 subscript is in the ionic formula because we need two cl −. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Those of most metals are ionic. For example, sodium chloride melts at 801 °c and boils at 1413 °c. Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. It is defined as being the charge that an atom would have if all bonds were ionic.
from www.elevise.co.uk
Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; The 2 subscript is in the ionic formula because we need two cl −. Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Those of most metals are ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. It is defined as being the charge that an atom would have if all bonds were ionic. For example, sodium chloride melts at 801 °c and boils at 1413 °c.
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise
Chlorine Is Ionic For example, sodium chloride melts at 801 °c and boils at 1413 °c. It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. For example, sodium chloride melts at 801 °c and boils at 1413 °c. The 2 subscript is in the ionic formula because we need two cl −. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Those of most metals are ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. The chlorine is in the form of a negatively charged ion, not the neutral element. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides;
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; For example, sodium chloride melts at 801 °c and boils at 1413 °c. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. The chlorine is in the form of a negatively charged. Chlorine Is Ionic.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Is Ionic Uncombined elements have an oxidation state of 0. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; It is defined as being the charge that an atom would have if all bonds were ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a. Chlorine Is Ionic.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Is Ionic It is defined as being the charge that an atom would have if all bonds were ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Uncombined elements have an oxidation state. Chlorine Is Ionic.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Is Ionic For example, sodium chloride melts at 801 °c and boils at 1413 °c. The chlorine is in the form of a negatively charged ion, not the neutral element. Uncombined elements have an oxidation state of 0. Those of most metals are ionic. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Chlorine combines. Chlorine Is Ionic.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Chlorine Is Ionic For example, sodium chloride melts at 801 °c and boils at 1413 °c. The 2 subscript is in the ionic formula because we need two cl −. It is defined as being the charge that an atom would have if all bonds were ionic. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.. Chlorine Is Ionic.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; The chlorine is in the form of a negatively charged ion, not the neutral element. It is defined as being the charge that an atom would have if all bonds were ionic. For example, sodium chloride melts at 801 °c and boils at 1413. Chlorine Is Ionic.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Is Ionic The chlorine is in the form of a negatively charged ion, not the neutral element. Uncombined elements have an oxidation state of 0. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; For example, in the reaction. Chlorine Is Ionic.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6315263 Chlorine Is Ionic The chlorine is in the form of a negatively charged ion, not the neutral element. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; It is defined as being the charge that an atom would have if all bonds were ionic. For example, in the reaction of na (sodium) and cl (chlorine), each. Chlorine Is Ionic.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine Is Ionic For example, sodium chloride melts at 801 °c and boils at 1413 °c. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. For example, in the reaction of na (sodium). Chlorine Is Ionic.
From www.dreamstime.com
Chlorine Chemical Element Symbol from Periodic Table Stock Illustration Chlorine Is Ionic For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. The chlorine is in the form of a negatively charged ion, not the neutral element. The 2 subscript is in the ionic formula because we need two cl −. Chlorine combines with almost all the elements, except for the. Chlorine Is Ionic.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. For example, sodium chloride melts at 801 °c and boils at 1413 °c. The chlorine is in the form of a negatively charged. Chlorine Is Ionic.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Is Ionic The chlorine is in the form of a negatively charged ion, not the neutral element. Those of most metals are ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. For example, sodium chloride melts at 801 °c and boils at 1413 °c. It is defined as being. Chlorine Is Ionic.
From pngtree.com
An Icon Showing The Chemical Chlorine Atomic Periodic Chemistry Vector Chlorine Is Ionic Those of most metals are ionic. The chlorine is in the form of a negatively charged ion, not the neutral element. The 2 subscript is in the ionic formula because we need two cl −. Uncombined elements have an oxidation state of 0. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For. Chlorine Is Ionic.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chlorine Is Ionic Those of most metals are ionic. Uncombined elements have an oxidation state of 0. The 2 subscript is in the ionic formula because we need two cl −. For example, sodium chloride melts at 801 °c and boils at 1413 °c. The chlorine is in the form of a negatively charged ion, not the neutral element. Ionic compounds are solids. Chlorine Is Ionic.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chlorine Is Ionic Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. For example, sodium chloride melts at 801 °c and boils at 1413 °c. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; The 2 subscript is in the ionic formula. Chlorine Is Ionic.
From depositphotos.com
Ionic Molecular Compounds Classification Pure Substances Atomic Chlorine Is Ionic Those of most metals are ionic. For example, sodium chloride melts at 801 °c and boils at 1413 °c. Uncombined elements have an oxidation state of 0. The 2 subscript is in the ionic formula because we need two cl −. The chlorine is in the form of a negatively charged ion, not the neutral element. Ionic compounds are solids. Chlorine Is Ionic.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Is Ionic Those of most metals are ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Uncombined elements have an oxidation state of 0. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; The chlorine is in the form of a negatively. Chlorine Is Ionic.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Is Ionic For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. The chlorine is in the form of a negatively charged ion, not the neutral element. The 2 subscript is in the ionic formula because we need two cl −. Uncombined elements have an oxidation state of 0. Chlorine combines. Chlorine Is Ionic.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Is Ionic Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. The chlorine is in the form of a negatively charged ion, not the neutral element. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; It is defined as being the charge that an atom would have if. Chlorine Is Ionic.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Is Ionic Uncombined elements have an oxidation state of 0. Those of most metals are ionic. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom. Chlorine Is Ionic.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Those of most metals are ionic. Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. It is defined as being the charge that an atom would have if all bonds. Chlorine Is Ionic.
From www.alamy.com
Chlorine chemical element with first ionization energy, atomic mass and Chlorine Is Ionic It is defined as being the charge that an atom would have if all bonds were ionic. The chlorine is in the form of a negatively charged ion, not the neutral element. Those of most metals are ionic. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Ionic compounds are solids that typically. Chlorine Is Ionic.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chlorine Is Ionic For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; It is defined as being the charge that an atom would have if all bonds were ionic. Ionic compounds are solids that typically. Chlorine Is Ionic.
From www.youtube.com
Is Cl2 (Chlorine gas) ionic or covalent? YouTube Chlorine Is Ionic Uncombined elements have an oxidation state of 0. Those of most metals are ionic. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. The chlorine is in the form of a negatively. Chlorine Is Ionic.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Is Ionic Those of most metals are ionic. Uncombined elements have an oxidation state of 0. The 2 subscript is in the ionic formula because we need two cl −. The chlorine is in the form of a negatively charged ion, not the neutral element. For example, sodium chloride melts at 801 °c and boils at 1413 °c. It is defined as. Chlorine Is Ionic.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Chlorine Is Ionic Uncombined elements have an oxidation state of 0. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Those of most metals are ionic. The chlorine is in the form of a negatively charged ion, not the neutral element. The 2 subscript is in the ionic formula because we need two cl −. For. Chlorine Is Ionic.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Uncombined elements have an oxidation state of 0. Those of most metals are ionic. For example, sodium chloride melts at 801 °c and boils at 1413 °c. The. Chlorine Is Ionic.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Is Ionic Uncombined elements have an oxidation state of 0. The 2 subscript is in the ionic formula because we need two cl −. For example, sodium chloride melts at 801 °c and boils at 1413 °c. It is defined as being the charge that an atom would have if all bonds were ionic. For example, in the reaction of na (sodium). Chlorine Is Ionic.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Uncombined elements have an oxidation state of 0. Those of most metals are ionic. The 2 subscript is in the ionic formula because we need two cl −. It is defined as being the charge that an atom would have if all bonds were. Chlorine Is Ionic.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Is Ionic Uncombined elements have an oxidation state of 0. The chlorine is in the form of a negatively charged ion, not the neutral element. Those of most metals are ionic. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801 °c and boils at 1413 °c. Chlorine combines. Chlorine Is Ionic.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Is Ionic Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Uncombined elements have an oxidation state of 0. Those of most metals are ionic. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; For example, sodium chloride melts at 801 °c and boils at 1413 °c. The. Chlorine Is Ionic.
From sciencenotes.org
Printable Periodic Table Element Symbols Chlorine Is Ionic Uncombined elements have an oxidation state of 0. For example, sodium chloride melts at 801 °c and boils at 1413 °c. Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; Those of most metals are ionic. The 2 subscript is in the ionic formula because we need two cl −. The chlorine is. Chlorine Is Ionic.
From saylordotorg.github.io
Ionic Bonding and Simple Ionic Compounds Chlorine Is Ionic For example, sodium chloride melts at 801 °c and boils at 1413 °c. Uncombined elements have an oxidation state of 0. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Those of. Chlorine Is Ionic.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration Chlorine Is Ionic Chlorine combines with almost all the elements, except for the lighter noble gases, to give chlorides; It is defined as being the charge that an atom would have if all bonds were ionic. Those of most metals are ionic. For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom.. Chlorine Is Ionic.
From ar.inspiredpencil.com
Chloride Ion Chlorine Is Ionic For example, in the reaction of na (sodium) and cl (chlorine), each cl atom takes one electron from a na atom. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. The 2 subscript is in the ionic formula because we need two cl −. For example, sodium chloride melts at 801 °c and. Chlorine Is Ionic.