Magnesium Hydroxide Weak Base . I've seen some sources say magnesium hydroxide is a strong base, and. I'm a bit puzzled about the kb of magnesium hydroxide. It is a weak base and has a ph value of 10.5 when dissolved in water. A weak base is one which doesn't convert fully into hydroxide ions in solution. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). When a weak base reacts with water, the position of. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Is magnesium hydroxide a weak base? It is used as an antacid to. When heated, magnesium hydroxide decomposes to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and.
from www.numerade.com
When a weak base reacts with water, the position of. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Is magnesium hydroxide a weak base? It is used as an antacid to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. I've seen some sources say magnesium hydroxide is a strong base, and. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. It is a weak base and has a ph value of 10.5 when dissolved in water. I'm a bit puzzled about the kb of magnesium hydroxide.
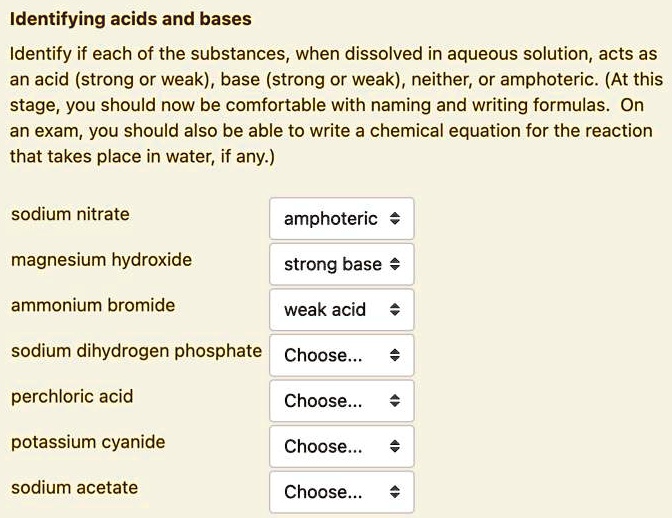
SOLVED Identifying acids and bases Identify if each of the substances
Magnesium Hydroxide Weak Base It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. When a weak base reacts with water, the position of. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). I'm a bit puzzled about the kb of magnesium hydroxide. Is magnesium hydroxide a weak base? It is a weak base and has a ph value of 10.5 when dissolved in water. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. I've seen some sources say magnesium hydroxide is a strong base, and. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. A weak base is one which doesn't convert fully into hydroxide ions in solution. When heated, magnesium hydroxide decomposes to. It is used as an antacid to.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2167333 Magnesium Hydroxide Weak Base I've seen some sources say magnesium hydroxide is a strong base, and. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. I'm a bit puzzled about the kb of magnesium hydroxide. Is magnesium hydroxide a weak base? It is a weak base and has a ph value of 10.5. Magnesium Hydroxide Weak Base.
From www.difference.wiki
Magnesium Oxide vs. Magnesium Hydroxide What’s the Difference? Magnesium Hydroxide Weak Base Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. Is magnesium hydroxide a weak base? It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. Magnesium hydroxide is a chemical compound with the. Magnesium Hydroxide Weak Base.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Magnesium Hydroxide Weak Base When a weak base reacts with water, the position of. Is magnesium hydroxide a weak base? Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. It is a weak base and has a ph value of 10.5 when dissolved in water. I'm a bit puzzled about the kb of. Magnesium Hydroxide Weak Base.
From slideplayer.com
WarmUp What is an acid? What is a base? ppt download Magnesium Hydroxide Weak Base It is used as an antacid to. A weak base is one which doesn't convert fully into hydroxide ions in solution. I'm a bit puzzled about the kb of magnesium hydroxide. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. Is magnesium hydroxide. Magnesium Hydroxide Weak Base.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Weak Base It is used as an antacid to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. I'm a bit puzzled about the kb of magnesium hydroxide. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). A weak base is one which. Magnesium Hydroxide Weak Base.
From www.researchgate.net
(a) Molecular structure diagram of magnesium hydroxide (b) Crystal Magnesium Hydroxide Weak Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. A weak base is one which doesn't convert fully into hydroxide ions in solution. I'm a bit. Magnesium Hydroxide Weak Base.
From acornwater.ie
Magnesium Hydroxide pH Correction Acorn Water Magnesium Hydroxide Weak Base I'm a bit puzzled about the kb of magnesium hydroxide. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. It is used as an antacid to. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It looks like a weak base because it is only. Magnesium Hydroxide Weak Base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Magnesium Hydroxide Weak Base Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. When heated, magnesium hydroxide decomposes to. When a weak base reacts with water, the position of. A weak base is one which doesn't convert fully into hydroxide ions in solution. Unlike lime, it is also much more difficult to treat. Magnesium Hydroxide Weak Base.
From www.slideserve.com
PPT Acid Base Theories 19.1 PowerPoint Presentation, free download Magnesium Hydroxide Weak Base When heated, magnesium hydroxide decomposes to. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. It is used as an antacid to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. When a weak base reacts with water, the position of. It. Magnesium Hydroxide Weak Base.
From tibetmag.com
Advantages and disadvantages of the process of preparing magnesium Magnesium Hydroxide Weak Base It is a weak base and has a ph value of 10.5 when dissolved in water. I've seen some sources say magnesium hydroxide is a strong base, and. When heated, magnesium hydroxide decomposes to. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). Were magnesium hydroxide a weak base, solutions of magnesium. Magnesium Hydroxide Weak Base.
From www.slideserve.com
PPT Common formulae PowerPoint Presentation, free download ID5978308 Magnesium Hydroxide Weak Base A weak base is one which doesn't convert fully into hydroxide ions in solution. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. I've seen some sources say magnesium hydroxide is a strong base, and. I'm a bit puzzled about the kb of magnesium hydroxide. It looks like a weak base because it. Magnesium Hydroxide Weak Base.
From www.slideserve.com
PPT Chemistry 102(01) Fall 2010 PowerPoint Presentation, free Magnesium Hydroxide Weak Base Is magnesium hydroxide a weak base? A weak base is one which doesn't convert fully into hydroxide ions in solution. When heated, magnesium hydroxide decomposes to. I'm a bit puzzled about the kb of magnesium hydroxide. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). When a weak base reacts with water,. Magnesium Hydroxide Weak Base.
From slideplayer.com
UNDERSTANDING ACIDS and ALKALIS ppt download Magnesium Hydroxide Weak Base Is magnesium hydroxide a weak base? It is used as an antacid to. I'm a bit puzzled about the kb of magnesium hydroxide. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. It is a weak base and has a ph value of 10.5 when dissolved in water. When heated, magnesium hydroxide decomposes. Magnesium Hydroxide Weak Base.
From www.numerade.com
SOLVED Identifying acids and bases Identify if each of the substances Magnesium Hydroxide Weak Base It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. When a weak base reacts with water, the position of. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. A weak base is one which doesn't convert. Magnesium Hydroxide Weak Base.
From slideplayer.com
Ch. 15 Acids & Bases ACIDS Have a H+ ion (Hydronium ion) Corrosive Magnesium Hydroxide Weak Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It is a weak base and has a ph value of 10.5 when dissolved in water. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. I've. Magnesium Hydroxide Weak Base.
From www.globeclad.com
Magnesium Hydroxide Structure Globeclad Fire Resistant Facades Magnesium Hydroxide Weak Base It is a weak base and has a ph value of 10.5 when dissolved in water. It is used as an antacid to. I've seen some sources say magnesium hydroxide is a strong base, and. A weak base is one which doesn't convert fully into hydroxide ions in solution. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly. Magnesium Hydroxide Weak Base.
From byjus.com
Solubility product of magnesium Hydroxide is 4 10 12 . The number of Magnesium Hydroxide Weak Base Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. It is used as an antacid to. A weak base is one which doesn't convert fully into hydroxide ions in solution. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). When a. Magnesium Hydroxide Weak Base.
From www.garrisonminerals.com
Magnesium Hydroxide vs. Caustic Soda in Wastewater Treatment Magnesium Hydroxide Weak Base I've seen some sources say magnesium hydroxide is a strong base, and. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It is used as an antacid to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. It is a weak. Magnesium Hydroxide Weak Base.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium Hydroxide Weak Base It is used as an antacid to. Is magnesium hydroxide a weak base? I'm a bit puzzled about the kb of magnesium hydroxide. It is a weak base and has a ph value of 10.5 when dissolved in water. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Were magnesium hydroxide a weak. Magnesium Hydroxide Weak Base.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Weak Base I've seen some sources say magnesium hydroxide is a strong base, and. It is used as an antacid to. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. I'm a bit puzzled about the kb of magnesium hydroxide. Is magnesium hydroxide a weak base? When heated, magnesium hydroxide decomposes. Magnesium Hydroxide Weak Base.
From chemygostar.com
Magnesium hydroxide (applications and synthesis methods) شرکت شیمی Magnesium Hydroxide Weak Base Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). It is used as an antacid to. It is a weak base and has a ph value of 10.5 when dissolved in water. I've seen some sources say magnesium hydroxide is a strong base, and. Were magnesium hydroxide a weak base, solutions of. Magnesium Hydroxide Weak Base.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide Weak Base It is used as an antacid to. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Is magnesium hydroxide a weak base? Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. It is a weak base and has a ph value of. Magnesium Hydroxide Weak Base.
From www.youtube.com
Is Mg(OH)2, Magnesium Hydroxide, an Acid, Base, or Neutral? YouTube Magnesium Hydroxide Weak Base It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. It is used as an antacid to. When heated, magnesium hydroxide decomposes to. When a weak base reacts with water, the position of. Is magnesium hydroxide a weak base? Magnesium hydroxide is a chemical. Magnesium Hydroxide Weak Base.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Magnesium Hydroxide Weak Base Is magnesium hydroxide a weak base? It is a weak base and has a ph value of 10.5 when dissolved in water. I've seen some sources say magnesium hydroxide is a strong base, and. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). A weak base is one which doesn't convert fully. Magnesium Hydroxide Weak Base.
From www.youtube.com
Magnesium hydroxide is a weak base cbseboard class10 acidsandbases Magnesium Hydroxide Weak Base I've seen some sources say magnesium hydroxide is a strong base, and. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. When heated, magnesium hydroxide decomposes to. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. I'm a. Magnesium Hydroxide Weak Base.
From slidetodoc.com
Properties of Acids and Bases Property Acid Base Magnesium Hydroxide Weak Base It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide. Magnesium Hydroxide Weak Base.
From www.tradewheel.com
Buy Magnesium Hydroxide from Hangzhou Shengmagnesium New Materials Co Magnesium Hydroxide Weak Base A weak base is one which doesn't convert fully into hydroxide ions in solution. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. Is magnesium hydroxide a weak base? Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. When a weak base. Magnesium Hydroxide Weak Base.
From www.alamy.com
3D image of Magnesium hydroxide skeletal formula molecular chemical Magnesium Hydroxide Weak Base It is a weak base and has a ph value of 10.5 when dissolved in water. A weak base is one which doesn't convert fully into hydroxide ions in solution. Were magnesium hydroxide a weak base, solutions of magnesium ion in water would hydrolyze in water removing hydroxide ions and. I've seen some sources say magnesium hydroxide is a strong. Magnesium Hydroxide Weak Base.
From www.youtube.com
Magnesium hydroxide Meaning YouTube Magnesium Hydroxide Weak Base When a weak base reacts with water, the position of. It is a weak base and has a ph value of 10.5 when dissolved in water. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. Unlike lime, it is also much more difficult. Magnesium Hydroxide Weak Base.
From slideplayer.com
Pharmacotherapy of peptic ulcer ppt download Magnesium Hydroxide Weak Base It is used as an antacid to. A weak base is one which doesn't convert fully into hydroxide ions in solution. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). Is magnesium hydroxide a weak base? I'm a bit puzzled about the kb of magnesium hydroxide. When heated, magnesium hydroxide decomposes to.. Magnesium Hydroxide Weak Base.
From www.slideshare.net
Acids and Bases Magnesium Hydroxide Weak Base I've seen some sources say magnesium hydroxide is a strong base, and. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. When a weak base reacts with water, the position of. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known. Magnesium Hydroxide Weak Base.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Magnesium Hydroxide Weak Base It is used as an antacid to. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. Is magnesium hydroxide a weak base? A weak base is one which doesn't convert fully into hydroxide ions in solution. I'm a bit puzzled about the kb of magnesium hydroxide. Were magnesium hydroxide a weak base, solutions. Magnesium Hydroxide Weak Base.
From www.researchgate.net
DRIFT spectra for magnesium hydroxide, hydrotalcite and hydromagnesite Magnesium Hydroxide Weak Base It is a weak base and has a ph value of 10.5 when dissolved in water. Magnesium hydroxide is a chemical compound with the formula mg(oh)2, commonly known as milk of magnesia. When heated, magnesium hydroxide decomposes to. A weak base is one which doesn't convert fully into hydroxide ions in solution. Were magnesium hydroxide a weak base, solutions of. Magnesium Hydroxide Weak Base.
From chemistrytalk.org
Weak Acids and Weak Bases ChemTalk Magnesium Hydroxide Weak Base It is a weak base and has a ph value of 10.5 when dissolved in water. When a weak base reacts with water, the position of. I've seen some sources say magnesium hydroxide is a strong base, and. Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (naoh). I'm a bit puzzled about. Magnesium Hydroxide Weak Base.
From www.quarkochem.com
Magnesium hydroxide Quarko Magnesium Hydroxide Weak Base Is magnesium hydroxide a weak base? I've seen some sources say magnesium hydroxide is a strong base, and. It looks like a weak base because it is only sparingly soluble in water, but the portion that does dissolve acts like a dilute solution of. When heated, magnesium hydroxide decomposes to. When a weak base reacts with water, the position of.. Magnesium Hydroxide Weak Base.