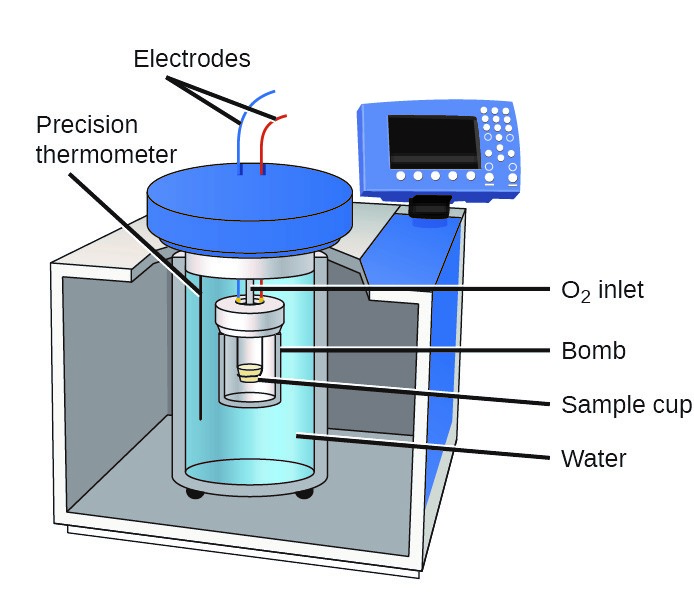
Bomb Calorimeter Role . In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bucket or container for holding the bomb in a measured quantity of water,. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Four essential parts are required in any bomb calorimeter: Its construction typically involves a. The consequence of the calculation is called the amount of combustion, calorification, or btu. In the calorimeter use of a strong.
from pressbooks.calstate.edu
Its construction typically involves a. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bucket or container for holding the bomb in a measured quantity of water,. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Four essential parts are required in any bomb calorimeter:
3.1 Calorimetry Nutrition and Physical Fitness
Bomb Calorimeter Role Four essential parts are required in any bomb calorimeter: Its construction typically involves a. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Four essential parts are required in any bomb calorimeter: In the calorimeter use of a strong. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. A bucket or container for holding the bomb in a measured quantity of water,. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A bomb or vessel in which the combustible charges can be burned.
From www.slideserve.com
PPT Chapter 8 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Role Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. The consequence of the calculation is called the amount of combustion, calorification, or btu. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter. Bomb Calorimeter Role.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. In the calorimeter use of a strong. The consequence of the calculation is called the amount of combustion, calorification,. Bomb Calorimeter Role.
From www.slideshare.net
The Role of Bomb Calorimeter Manufacturers in Scientific Advancements PDF Bomb Calorimeter Role Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A bucket or container for holding the bomb in a measured quantity of water,. A bomb calorimeter is a scientific. Bomb Calorimeter Role.
From www.youtube.com
Bomb Calorimeter Working and Principle of Bomb Calorimeter GCV of Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Its construction typically involves a. In the calorimeter use of a strong. A bomb or vessel in which the combustible charges can be burned. Bomb calorimeter. Bomb Calorimeter Role.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Role A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter is a device used to measure. Bomb Calorimeter Role.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Role A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Its construction typically involves a. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and. Bomb Calorimeter Role.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Bomb Calorimeter Role A bomb or vessel in which the combustible charges can be burned. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. The reaction takes place in a closed space known as the calorimeter. In the calorimeter use of a strong. A bucket or container for holding the bomb in. Bomb Calorimeter Role.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Role The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Its construction. Bomb Calorimeter Role.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Role A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. The consequence of the calculation is called the amount of combustion, calorification, or btu. The calorimeter is made of austenitic steel. Bomb Calorimeter Role.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Bomb Calorimeter Role A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a measured quantity of water,. A bomb calorimeter is used to measure, under controlled conditions,. Bomb Calorimeter Role.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Role In the calorimeter use of a strong. Four essential parts are required in any bomb calorimeter: The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The. Bomb Calorimeter Role.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimeter Role A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. In the calorimeter. Bomb Calorimeter Role.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Role Its construction typically involves a. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. A bomb or vessel in which the combustible charges can be burned. A bomb. Bomb Calorimeter Role.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Role A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Bomb calorimeter an apparatus. Bomb Calorimeter Role.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. In the calorimeter use of a strong. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The reaction takes place in a closed space known as. Bomb Calorimeter Role.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. Four essential parts are required in any bomb calorimeter: In the calorimeter use of a strong. The reaction takes place in a closed space known as the calorimeter. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is a device used. Bomb Calorimeter Role.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring Bomb Calorimeter Role The reaction takes place in a closed space known as the calorimeter. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb or vessel in which the combustible charges can be burned. In the calorimeter use of. Bomb Calorimeter Role.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter Role The reaction takes place in a closed space known as the calorimeter. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bucket or container for holding the bomb in a measured quantity of water,. Its. Bomb Calorimeter Role.
From www.youtube.com
MECHANICAL ENGINEERING THE BOMB CALORIMETER IS AN APPARATUS TO MEASURE Bomb Calorimeter Role The reaction takes place in a closed space known as the calorimeter. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured.. Bomb Calorimeter Role.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Role Its construction typically involves a. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and. Bomb Calorimeter Role.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Bomb Calorimeter Role In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. Its construction typically involves a. The consequence of the. Bomb Calorimeter Role.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Role Its construction typically involves a. The consequence of the calculation is called the amount of combustion, calorification, or btu. The reaction takes place in a closed space known as the calorimeter. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured. A bomb or vessel in which the. Bomb Calorimeter Role.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Bomb Calorimeter Role In the calorimeter use of a strong. A bomb or vessel in which the combustible charges can be burned. The reaction takes place in a closed space known as the calorimeter. A bucket or container for holding the bomb in a measured quantity of water,. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables. Bomb Calorimeter Role.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Bomb Calorimeter Role A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. Its construction typically involves a. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is a scientific instrument. Bomb Calorimeter Role.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Role In the calorimeter use of a strong. A bucket or container for holding the bomb in a measured quantity of water,. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured.. Bomb Calorimeter Role.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Role A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: Its construction typically involves a. A bomb calorimeter is a. Bomb Calorimeter Role.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Role A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The consequence of the calculation. Bomb Calorimeter Role.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. The reaction takes place in a closed space known as the calorimeter. Four essential parts are required in any bomb calorimeter: The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. A bomb calorimeter is used. Bomb Calorimeter Role.
From wps.prenhall.com
Media Portfolio Bomb Calorimeter Role A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bucket or container for holding the bomb in a measured quantity of water,. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a. Bomb Calorimeter Role.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Role The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. In the calorimeter use of a strong. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The reaction takes place in a closed space known as the calorimeter. Its construction typically involves a. The consequence of the calculation. Bomb Calorimeter Role.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Bomb Calorimeter Role A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Four essential parts are required in any bomb calorimeter: In the process of. Bomb Calorimeter Role.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Role A bucket or container for holding the bomb in a measured quantity of water,. The reaction takes place in a closed space known as the calorimeter. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. Four essential parts are required in any bomb calorimeter: The consequence of the calculation is called the. Bomb Calorimeter Role.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Role Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of solutions and the. In the process of bomb calorimetry, the sample is rapidly combusted in oxygen at increased pressure, and the heat production is measured.. Bomb Calorimeter Role.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Role The reaction takes place in a closed space known as the calorimeter. The calorimeter is made of austenitic steel which provides considerable resistance to corrosion and enables it to withstand high pressure. Four essential parts are required in any bomb calorimeter: Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb or vessel in which the combustible. Bomb Calorimeter Role.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Role In the calorimeter use of a strong. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A bucket or container for holding the bomb in a measured quantity of water,. Its construction typically involves a. Bomb calorimeter an apparatus primarily. Bomb Calorimeter Role.