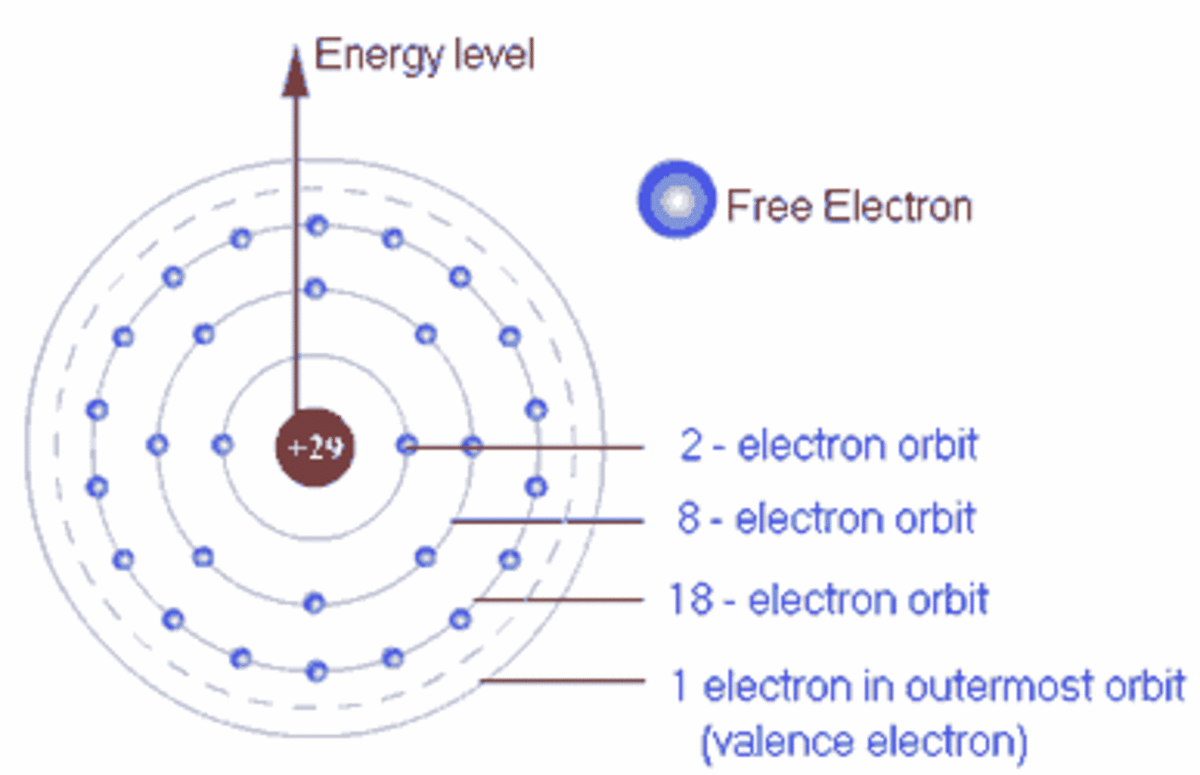
How Many Electrons Can Chlorine Hold . — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has 7 electrons in its valence shell, making it highly electronegative. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its.
from owlcation.com
in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Chlorine has 7 electrons in its valence shell, making it highly electronegative. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its.
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
How Many Electrons Can Chlorine Hold Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Chlorine has 7 electrons in its valence shell, making it highly electronegative.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology How Many Electrons Can Chlorine Hold — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. — chlorine has an atomic number of 17 with 2, 8,. How Many Electrons Can Chlorine Hold.
From courses.lumenlearning.com
Electrons Biology for Majors I How Many Electrons Can Chlorine Hold — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. in order to write the chlorine electron configuration we first need to know the. How Many Electrons Can Chlorine Hold.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Each atom. How Many Electrons Can Chlorine Hold.
From www.benjamin-mills.com
Electron configurations How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has 7 electrons in its valence shell, making it. How Many Electrons Can Chlorine Hold.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. . How Many Electrons Can Chlorine Hold.
From www.nagwa.com
Question Video Finding the Number of Additional Electrons an Electron How Many Electrons Can Chlorine Hold Chlorine has 7 electrons in its valence shell, making it highly electronegative. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the octet rule is based. How Many Electrons Can Chlorine Hold.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons How Many Electrons Can Chlorine Hold Chlorine has 7 electrons in its valence shell, making it highly electronegative. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.. How Many Electrons Can Chlorine Hold.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I How Many Electrons Can Chlorine Hold — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Chlorine has an atomic number of 17, which means it has 17 protons and therefore. How Many Electrons Can Chlorine Hold.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock How Many Electrons Can Chlorine Hold in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. — chlorine has an atomic number of 17 with 2, 8, and 7. How Many Electrons Can Chlorine Hold.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Chlorine has an atomic number of 17, which means it has 17 protons. How Many Electrons Can Chlorine Hold.
From littleeagles.edu.vn
How To Find The Protons, Neutrons And Electrons For Chlorine? How Many Electrons Can Chlorine Hold Chlorine has 7 electrons in its valence shell, making it highly electronegative. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. the. How Many Electrons Can Chlorine Hold.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Many Electrons Can Chlorine Hold the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in. How Many Electrons Can Chlorine Hold.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. . How Many Electrons Can Chlorine Hold.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Chlorine has 7 electrons in its valence shell, making it highly electronegative. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas.. How Many Electrons Can Chlorine Hold.
From download4update.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Each atom just needs to adjust 1 electron to have a completely. How Many Electrons Can Chlorine Hold.
From www.expii.com
Ions — Definition & Overview Expii How Many Electrons Can Chlorine Hold the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. — chlorine has high electronegativity and higher electron affinity, so it. How Many Electrons Can Chlorine Hold.
From nl.depositphotos.com
Diagram weergave van het element natrium vectorafbeelding door How Many Electrons Can Chlorine Hold — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has 7 electrons in its valence shell, making it highly electronegative. Chlorine. How Many Electrons Can Chlorine Hold.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Chlorine has 7 electrons in its valence shell, making it highly electronegative.. How Many Electrons Can Chlorine Hold.
From dxofcoqqf.blob.core.windows.net
How Many Valence Electrons Are There In Chlorine at Candace Kelly blog How Many Electrons Can Chlorine Hold Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. . How Many Electrons Can Chlorine Hold.
From slideplayer.com
CHEMISTRY Chapter ppt download How Many Electrons Can Chlorine Hold — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration. How Many Electrons Can Chlorine Hold.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons How Many Electrons Can Chlorine Hold — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has 7 electrons in its valence shell, making it highly electronegative. . How Many Electrons Can Chlorine Hold.
From www.showme.com
Chem Chpt. 1 How many electrons fit in each shell? Science ShowMe How Many Electrons Can Chlorine Hold — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Chlorine has an atomic number of 17, which means it has 17 protons and. How Many Electrons Can Chlorine Hold.
From www.youtube.com
Chlorine Electron Configuration YouTube How Many Electrons Can Chlorine Hold — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Each atom just needs to adjust 1 electron to have a completely. How Many Electrons Can Chlorine Hold.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Each atom just needs to adjust 1 electron to have a completely filled valence shell. How Many Electrons Can Chlorine Hold.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions How Many Electrons Can Chlorine Hold Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Each atom just needs to adjust. How Many Electrons Can Chlorine Hold.
From www.nagwa.com
Question Video Identifying the Statement That Describes How a Single How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. the octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. Chlorine has 7 electrons in its valence shell, making it highly electronegative.. How Many Electrons Can Chlorine Hold.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind How Many Electrons Can Chlorine Hold in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Chlorine has 7 electrons in its valence shell, making it highly electronegative. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. Each atom just. How Many Electrons Can Chlorine Hold.
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce How Many Electrons Can Chlorine Hold in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine has an atomic number of 17 with 2, 8, and 7. How Many Electrons Can Chlorine Hold.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other How Many Electrons Can Chlorine Hold Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in. How Many Electrons Can Chlorine Hold.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica How Many Electrons Can Chlorine Hold — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Chlorine has 7 electrons in its valence shell, making it highly electronegative. —. How Many Electrons Can Chlorine Hold.
From material-properties.org
Chlorine Periodic Table and Atomic Properties How Many Electrons Can Chlorine Hold — chlorine has an atomic number of 17 with 2, 8, and 7 electrons in the k, l and m shells. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Each atom just needs to adjust 1 electron to have a completely filled valence shell. How Many Electrons Can Chlorine Hold.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons How Many Electrons Can Chlorine Hold in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. — chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. the octet rule is based on the fact that each valence orbital (typically,. How Many Electrons Can Chlorine Hold.
From byjus.com
In the electron dot structure, the valence shell electrons are How Many Electrons Can Chlorine Hold — chlorine, cl, is located in period 3, group 17 of the periodic table, and has an atomic number equal to 17. Each atom just needs to adjust 1 electron to have a completely filled valence shell and attain a configuration similar to the closest noble gas. Chlorine has 7 electrons in its valence shell, making it highly electronegative.. How Many Electrons Can Chlorine Hold.
From ameblo.jp
Chlorine Electrons exarepec1973のブログ How Many Electrons Can Chlorine Hold Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the octet rule is based on the fact that each valence orbital (typically, one ns and three. How Many Electrons Can Chlorine Hold.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision How Many Electrons Can Chlorine Hold Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the octet rule is based on the fact that each valence orbital (typically, one ns and three. How Many Electrons Can Chlorine Hold.