Neutralization Lab Report . The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: A strong acid/strong base neutralization and a weak acid/strong base neutralization. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. To measure, using a calorimeter, the energy change accompanying neutralization reactions.
from www.pdfprof.com
The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Ha + naoh fi naa +. Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. To measure, using a calorimeter, the energy change accompanying neutralization reactions. A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions:
experiment 25 calorimetry lab report
Neutralization Lab Report Ha + naoh fi naa +. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution: To measure, using a calorimeter, the energy change accompanying neutralization reactions. Ha + naoh fi naa +.
From studylib.net
Determining the Enthalpy of a Neutralization Reaction Work Sheet Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution: The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. To measure, using a. Neutralization Lab Report.
From www.studypool.com
SOLUTION CHEM 3H1 USTP Heat of Neutralization Lab Report Studypool Neutralization Lab Report Neutralization of hcl solution with naoh solution: A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: To measure, using a calorimeter, the energy change accompanying neutralization reactions. Ha + naoh fi naa +. Heat of neutralization purpose the purpose of this experiment. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report (1 week) (LCA) Enthalpy of Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. To measure, using a calorimeter, the energy change accompanying neutralization reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report Name Partner Student No Neutralization Lab Report Neutralization of hcl solution with naoh solution: A strong acid/strong base neutralization and a weak acid/strong base neutralization. Ha + naoh fi naa +. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its. Neutralization Lab Report.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Neutralization Lab Report A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Ha + naoh fi naa +.. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report 2024 Experiment 8. ( 1 week Neutralization Lab Report Neutralization of hcl solution with naoh solution: The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Ha + naoh fi naa +. A strong acid/strong base neutralization and a weak acid/strong base neutralization. To measure, using a calorimeter, the energy change accompanying neutralization reactions. Heat of neutralization purpose the purpose of this experiment. Neutralization Lab Report.
From www.aplustopper.com
What is the enthalpy of neutralization? A Plus Topper Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Ha + naoh fi naa +. Neutralization of hcl solution with naoh solution: To measure, using a calorimeter, the energy change accompanying neutralization reactions. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction.. Neutralization Lab Report.
From www.chegg.com
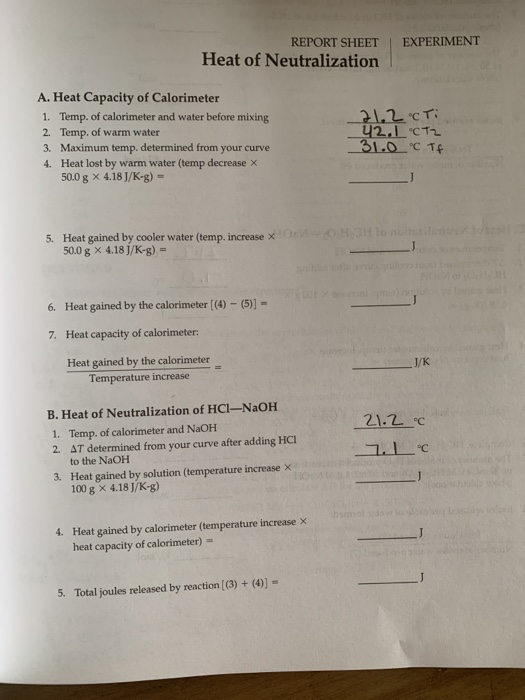
REPORT SHEET EXPERIMENT 28 Heat of Neutralization °C Neutralization Lab Report The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Ha + naoh fi naa +. Neutralization. Neutralization Lab Report.
From www.academia.edu
(DOC) EXPERIMENT 3 NEUTRALIZATION TITRATION OF STRONG ACID AND STRONG Neutralization Lab Report To measure, using a calorimeter, the energy change accompanying neutralization reactions. Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the.. Neutralization Lab Report.
From www.academia.edu
(DOC) Enthalpy of Neutralization Procedure/Experiment Jonniel Vince Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Ha + naoh fi naa +. A strong acid/strong base neutralization and a weak acid/strong base neutralization. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Neutralization of hcl solution with. Neutralization Lab Report.
From www.scribd.com
Naman Parikh Chemistry Lab Report On Enthalpy of Neutralization Neutralization Lab Report Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The purpose of this lab. Neutralization Lab Report.
From www.studocu.com
Enthalpy of neutralization lab Experiment 8. ( 1 week) (LCA) Enthalpy Neutralization Lab Report A strong acid/strong base neutralization and a weak acid/strong base neutralization. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The purpose of this lab is to. Neutralization Lab Report.
From www.chegg.com
Report Form, Experiment 9 Enthalpy of Neutralization Lab Report The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Ha + naoh fi naa +. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution: To measure, using a calorimeter, the energy change accompanying neutralization reactions. The purpose of. Neutralization Lab Report.
From www.chegg.com
Solved Name, Report Form, Experiment 9Enthalpy of Neutralization Lab Report A strong acid/strong base neutralization and a weak acid/strong base neutralization. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. To measure, using a calorimeter, the energy change accompanying neutralization reactions. Ha + naoh fi naa +. Neutralization of hcl solution with naoh solution: Heat of neutralization. Neutralization Lab Report.
From www.chegg.com
Solved nid Rase Neutralization Lab Report AcidBase Neutralization Lab Report A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction.. Neutralization Lab Report.
From www.pdfprof.com
experiment 25 calorimetry lab report Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Neutralization of hcl solution with naoh solution: To measure, using a calorimeter, the energy change accompanying neutralization reactions. A strong acid/strong base neutralization and. Neutralization Lab Report.
From www.studocu.com
Lab 6 report lab Thermochemistry Heat of Neutralization and Hess’s Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +.. Neutralization Lab Report.
From www.chegg.com
Date It . Name OUILLIRMLab Instructor Report Sheet An Neutralization Lab Report Neutralization of hcl solution with naoh solution: The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Ha + naoh fi naa +.. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report 1 Lab Section 021 Bench (on Neutralization Lab Report To measure, using a calorimeter, the energy change accompanying neutralization reactions. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: A strong acid/strong base neutralization and a weak acid/strong base neutralization. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the.. Neutralization Lab Report.
From studylib.net
Neutralization Lab Neutralization Lab Report Ha + naoh fi naa +. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: To measure, using a calorimeter, the energy change accompanying neutralization reactions. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The heat of neutralization that is lost. Neutralization Lab Report.
From studylib.net
Lab on Thermochemistry & Hess's Law Neutralization Lab Report Ha + naoh fi naa +. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: A strong acid/strong base neutralization and a weak acid/strong base neutralization. Neutralization of hcl solution with naoh solution:. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report Name Teni Ojedokun Partner Neutralization Lab Report The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. A strong acid/strong base neutralization and a weak acid/strong base neutralization.. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report Experiment 8. (1 week) (LCA Neutralization Lab Report The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. To measure, using a calorimeter, the energy change accompanying neutralization reactions. Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction.. Neutralization Lab Report.
From www.chegg.com
Solved all The Enthalpy of Neutralization of Phosphoric Acid Neutralization Lab Report Ha + naoh fi naa +. To measure, using a calorimeter, the energy change accompanying neutralization reactions. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: A strong acid/strong base neutralization and a weak acid/strong base neutralization. The heat of neutralization that is lost in the chemical reaction (the system) is gained by. Neutralization Lab Report.
From www.bartleby.com
Answered This the table data of Neutralization… bartleby Neutralization Lab Report Ha + naoh fi naa +. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Neutralization of hcl solution with naoh solution: To measure, using a calorimeter, the energy change accompanying neutralization reactions. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions:. Neutralization Lab Report.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Neutralization Lab Report Neutralization of hcl solution with naoh solution: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Ha + naoh fi naa +. To measure, using a calorimeter, the energy change accompanying neutralization reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by. Neutralization Lab Report.
From www.studypool.com
SOLUTION Lab report determination of enthalpy change of neutralization Neutralization Lab Report Ha + naoh fi naa +. To measure, using a calorimeter, the energy change accompanying neutralization reactions. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Neutralization of hcl solution with naoh solution: The heat of neutralization that is lost in the chemical reaction (the system) is gained by. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report Name Anika Anjum Partner Neutralization Lab Report Ha + naoh fi naa +. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Neutralization of hcl solution with naoh solution: A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions:. Neutralization Lab Report.
From www.studocu.com
The Enthalpy of Neutralization of Phosphoric Acid Worksheet Make sure Neutralization Lab Report Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. To measure, using a calorimeter, the energy change accompanying neutralization reactions. Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The heat of neutralization that is lost in the chemical reaction (the system) is gained by. Neutralization Lab Report.
From www.studocu.com
Enthalpy of Neutralization Lab Report Report Enthalpy of Neutralization Lab Report To measure, using a calorimeter, the energy change accompanying neutralization reactions. A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The heat of neutralization that is lost in the. Neutralization Lab Report.
From oneclass.com
OneClass Heat of Neutralization Lab Partner Date Reaction 1 Reaction 2 Neutralization Lab Report To measure, using a calorimeter, the energy change accompanying neutralization reactions. A strong acid/strong base neutralization and a weak acid/strong base neutralization. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Neutralization of. Neutralization Lab Report.
From www.studocu.com
Enthalpy of neutrilaztion Experiment 8. (1 week) (LCA) Enthalpy of Neutralization Lab Report Neutralization of hcl solution with naoh solution: Ha + naoh fi naa +. The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Heat of neutralization purpose the purpose of this experiment. Neutralization Lab Report.
From www.studocu.com
Lab 6 Neutralization Between Acid/Base Lab 6 Assignment Report Neutralization Lab Report Ha + naoh fi naa +. A strong acid/strong base neutralization and a weak acid/strong base neutralization. To measure, using a calorimeter, the energy change accompanying neutralization reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. The purpose of this lab is to use calorimetry concepts. Neutralization Lab Report.
From www.slideshare.net
Heat of Neutralization Lab Report PDF Neutralization Lab Report Ha + naoh fi naa +. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. A strong acid/strong base neutralization and a weak acid/strong base neutralization. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. To. Neutralization Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Neutralization Lab Report The purpose of this lab is to use calorimetry concepts to calculate enthalpy of 2 reactions: Ha + naoh fi naa +. Heat of neutralization purpose the purpose of this experiment is to determine the heat of reaction for a neutralization reaction. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter. Neutralization Lab Report.