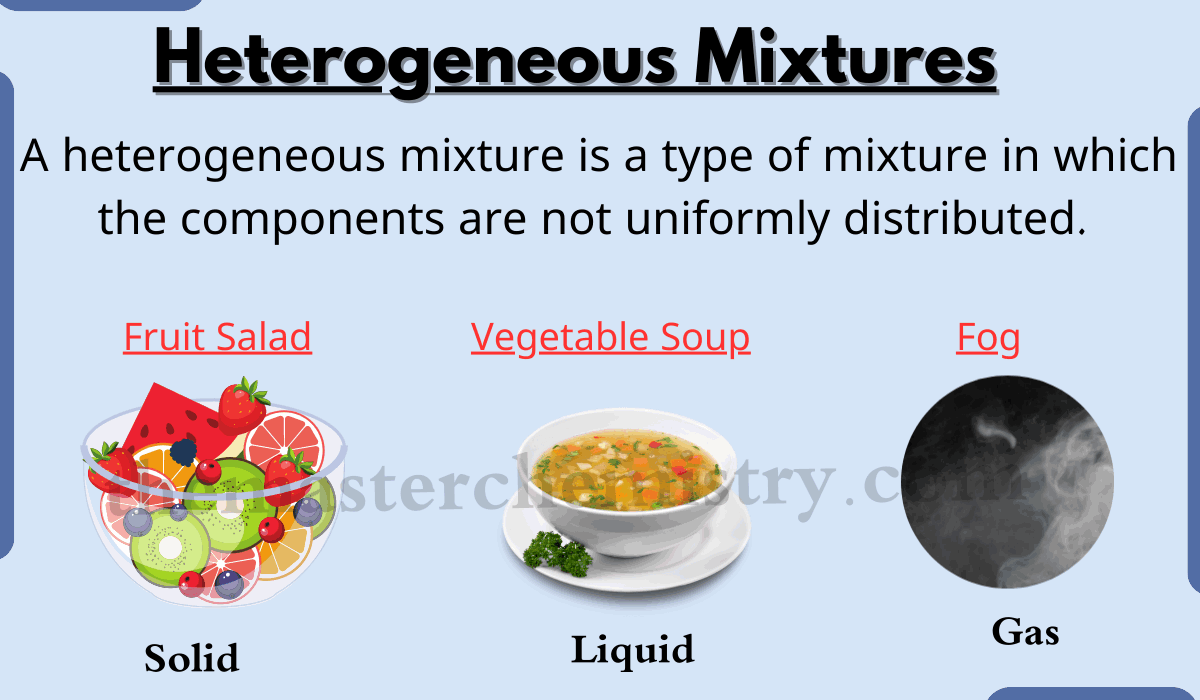
Is Table Salt An Element Compound Solution Or Heterogeneous Mixture . a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. These two elements can combine to form the compound,. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; All solutions are considered homogeneous because the. Think of oil and water mixed together: A heterogeneous mixture has a clear line between the two substances. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. the amount of salt in the salt water can vary from one sample to another. for example sodium is a soft shiny metal and chlorine is a pungent green gas.
from themasterchemistry.com
Think of oil and water mixed together: for example sodium is a soft shiny metal and chlorine is a pungent green gas. A heterogeneous mixture has a clear line between the two substances. the amount of salt in the salt water can vary from one sample to another. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. All solutions are considered homogeneous because the. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; These two elements can combine to form the compound,. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound.
Heterogeneous Mixture Example30 Fact Checked
Is Table Salt An Element Compound Solution Or Heterogeneous Mixture All solutions are considered homogeneous because the. A heterogeneous mixture has a clear line between the two substances. All solutions are considered homogeneous because the. Think of oil and water mixed together: classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. These two elements can combine to form the compound,. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. for example sodium is a soft shiny metal and chlorine is a pungent green gas. the amount of salt in the salt water can vary from one sample to another.
From 88guru.com
Mixtures & Compounds Types, Differences & Characteristics Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. Think of oil and water mixed together: the amount of salt in the salt water can vary. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.thoughtco.com
Chemical Composition of Table Salt Is Table Salt An Element Compound Solution Or Heterogeneous Mixture when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. A heterogeneous mixture has a clear line between the two substances. Think of oil and water mixed together: All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From cabinet.matttroy.net
Is Table Salt A Heterogeneous Mixture Solution Compound Or Element Matttroy Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. Think of oil and water mixed together: classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; a mixture is composed of two or more types of matter that can be present in varying amounts and can be. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From mavink.com
Venn Diagram Elements Compounds And Mixtures Is Table Salt An Element Compound Solution Or Heterogeneous Mixture when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; the amount of salt in the salt water can vary from one sample to another. A heterogeneous mixture has. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water. Homogeneous, heterogeneous Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. the amount of salt in the salt water can vary from one sample to another. classify. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.chemicals.co.uk
What is a Mixture in Chemistry? The Chemistry Blog Is Table Salt An Element Compound Solution Or Heterogeneous Mixture Think of oil and water mixed together: These two elements can combine to form the compound,. All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. for example sodium is a soft shiny metal and chlorine is a. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.numerade.com
SOLVED Classify each substance aS an element; compound, homogeneous mixture, Or heterogeneous Is Table Salt An Element Compound Solution Or Heterogeneous Mixture a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. the amount of salt in the salt water can vary from one sample to another.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From cabinet.matttroy.net
Is Table Salt A Heterogeneous Mixture Solution Compound Or Element Matttroy Is Table Salt An Element Compound Solution Or Heterogeneous Mixture classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; the amount of salt in the salt water can vary from one sample to another. These two elements can combine to form the compound,. Think of oil and water mixed together: a mixture is composed of two or. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From circuitdiagramalexandra.z5.web.core.windows.net
Element Compound Mixture Diagram Is Table Salt An Element Compound Solution Or Heterogeneous Mixture a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. for example sodium is a soft shiny metal and chlorine is a pungent green gas.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From themasterchemistry.com
Heterogeneous Mixture Example30 Fact Checked Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From elchoroukhost.net
Is Table Salt A Heterogeneous Mixture Solution Compound Or Element Elcho Table Is Table Salt An Element Compound Solution Or Heterogeneous Mixture for example sodium is a soft shiny metal and chlorine is a pungent green gas. A heterogeneous mixture has a clear line between the two substances. These two elements can combine to form the compound,. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. the amount of. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From ac2emsjcarmona.blogspot.com
6th Grade Science 1st Six Weeks (week 5) Pure Substances and Mixtures Is Table Salt An Element Compound Solution Or Heterogeneous Mixture for example sodium is a soft shiny metal and chlorine is a pungent green gas. All solutions are considered homogeneous because the. A heterogeneous mixture has a clear line between the two substances. the amount of salt in the salt water can vary from one sample to another. when two distinct elements are chemically combined—i.e., chemical bonds. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Matter Is Table Salt An Element Compound Solution Or Heterogeneous Mixture a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. the amount of salt in the salt water can vary from one sample to another. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From ar.inspiredpencil.com
Liquid Mixtures Examples Is Table Salt An Element Compound Solution Or Heterogeneous Mixture These two elements can combine to form the compound,. the amount of salt in the salt water can vary from one sample to another. Think of oil and water mixed together: when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. classify matter as an element, compound, homogeneous. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; A heterogeneous mixture has a clear line between the two substances. Think of oil and water mixed together: All solutions are considered homogeneous because. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From dxokefnjy.blob.core.windows.net
Table Salt Dissolved In Water Heterogeneous Or Homogeneous Mixture at Joe McCain blog Is Table Salt An Element Compound Solution Or Heterogeneous Mixture a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; for example sodium is a soft shiny metal and chlorine is a pungent green gas.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From elchoroukhost.net
Is Table Salt A Heterogeneous Mixture Solution Compound Or Element Elcho Table Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. Think of oil and water mixed together: All solutions are considered homogeneous because the. These two elements can combine to form the compound,. a mixture is composed of two or more types of matter that can be present in varying amounts and can. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples Is Table Salt An Element Compound Solution Or Heterogeneous Mixture Think of oil and water mixed together: for example sodium is a soft shiny metal and chlorine is a pungent green gas. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. All solutions are considered homogeneous because the. the amount of salt in. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Is Table Salt An Element Compound Solution Or Heterogeneous Mixture when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. Think of oil and water mixed together: classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; These two elements can combine to form the compound,. A heterogeneous mixture has. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From printablelistquinta.z21.web.core.windows.net
Homogeneous And Heterogeneous Lesson Plan Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. All solutions are considered homogeneous because the. Think of oil and water mixed together: These two elements can combine to form the compound,. A heterogeneous mixture has a clear line between the two substances. when two distinct elements are chemically combined—i.e., chemical bonds. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.nutriinspector.com
Is Salt A Mixture, Compound Or Element? Unraveling The Nature Of Salt Nutri Inspector Is Table Salt An Element Compound Solution Or Heterogeneous Mixture when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. for example sodium is a soft shiny metal and chlorine is a pungent green gas. These two elements can combine to form the compound,. a mixture is composed of two or more types of matter that can be. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Is Table Salt An Element Compound Solution Or Heterogeneous Mixture when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. for example sodium is a soft shiny metal and chlorine is a pungent green gas.. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From heimscience.weebly.com
Elements Compounds and Mixtures Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. All solutions are considered homogeneous because the. These two elements can combine to form the compound,. Think of oil and water mixed together: the amount of salt in. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From temperaturemaster.com
Is Table Salt a Compound, Mixture, or Solution? TempMaster Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. These two elements can combine to form the compound,. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. Think of oil and water mixed together: A heterogeneous mixture has a clear line between. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From herbalic79.blogspot.com
Element Compound Mixture Diagram Worksheet Herbalic Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; the amount of salt in the salt water can vary from one sample to another. Think of oil and water mixed together: a mixture is composed of. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From learningschooldodirniuo.z4.web.core.windows.net
Mixture Vs Substance Examples Is Table Salt An Element Compound Solution Or Heterogeneous Mixture These two elements can combine to form the compound,. All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. Think of oil and water mixed together: for example sodium is a soft shiny metal and chlorine is a. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From studylib.net
element, compound, heterogeneous mixture or homogeneous mixture Is Table Salt An Element Compound Solution Or Heterogeneous Mixture Think of oil and water mixed together: A heterogeneous mixture has a clear line between the two substances. These two elements can combine to form the compound,. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. a mixture is composed of two or more types of matter that. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.expii.com
Homogeneous vs. Heterogeneous Mixtures — A Comparison Expii Is Table Salt An Element Compound Solution Or Heterogeneous Mixture the amount of salt in the salt water can vary from one sample to another. All solutions are considered homogeneous because the. for example sodium is a soft shiny metal and chlorine is a pungent green gas. These two elements can combine to form the compound,. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From mungfali.com
Element Compound And Mixture Chart Is Table Salt An Element Compound Solution Or Heterogeneous Mixture classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. These two elements can combine to form the compound,. All solutions are considered homogeneous because the. the amount of. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From mungfali.com
10 Examples Of Heterogeneous Mixture Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; a mixture is composed of two or more. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.slideserve.com
PPT Classification of Matter PowerPoint Presentation, free download ID1549766 Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. for example sodium is a soft shiny metal and chlorine is a pungent green gas. All solutions are considered homogeneous because the. These two elements can combine to form the compound,. classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From www.len.com.ng
Types of Mixture Homogenous and Heterogeneous Mixtures Is Table Salt An Element Compound Solution Or Heterogeneous Mixture classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. Think of oil and water mixed together: All solutions are considered homogeneous because the. for. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From manualfixageundeployed.z13.web.core.windows.net
Mixture Of Compounds Diagram Is Table Salt An Element Compound Solution Or Heterogeneous Mixture A heterogeneous mixture has a clear line between the two substances. for example sodium is a soft shiny metal and chlorine is a pungent green gas. All solutions are considered homogeneous because the. a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. the. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From elchoroukhost.net
Is Table Salt A Compound Or Mixture Elcho Table Is Table Salt An Element Compound Solution Or Heterogeneous Mixture All solutions are considered homogeneous because the. Think of oil and water mixed together: a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. when two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. for. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.
From temperaturemaster.com
Is Table Salt a Compound, Mixture, or Solution? TempMaster Is Table Salt An Element Compound Solution Or Heterogeneous Mixture classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition; a mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by. A heterogeneous mixture has a clear line between the two substances. All solutions are considered homogeneous. Is Table Salt An Element Compound Solution Or Heterogeneous Mixture.