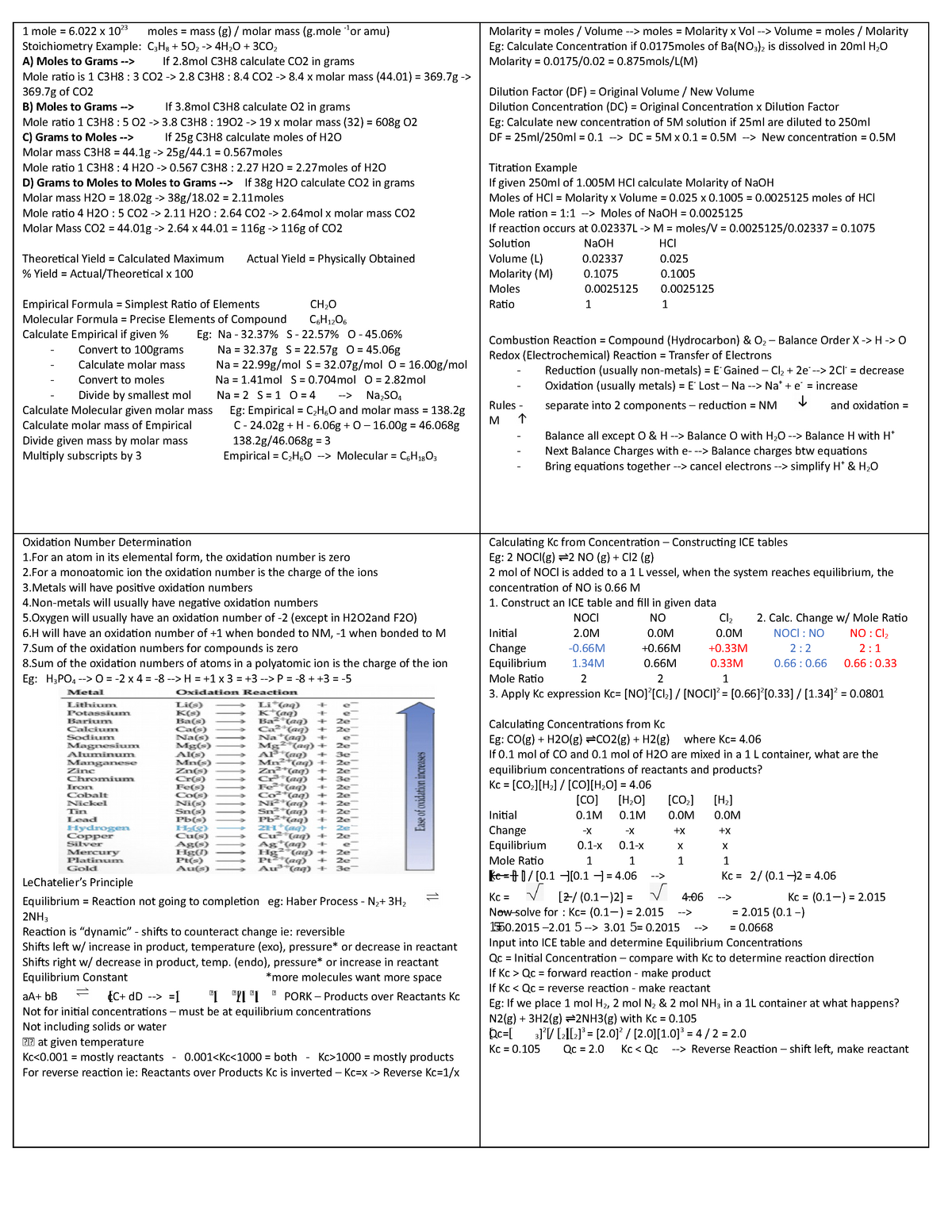
Titration Empirical Formula . At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Calculation of the empirical formula from mass data. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. Titration of a base with an acid. The simplest ratio of atoms of each element in a particular compound (for example, glucose: The empirical formula of the compound is \(\ce{fe_2o_3}\). The process of a titration problem follows the same process of any other stoichiometric problem. — write the empirical formula.
from www.studocu.com
At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. Titration of a base with an acid. The simplest ratio of atoms of each element in a particular compound (for example, glucose: Calculation of the empirical formula from mass data. — write the empirical formula. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. The empirical formula of the compound is \(\ce{fe_2o_3}\). The process of a titration problem follows the same process of any other stoichiometric problem. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the.
Stoichiometry Empirical and Molecular Formulas Equilibrium Titration Redox Reactions Studocu
Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). The empirical formula of the compound is \(\ce{fe_2o_3}\). The simplest ratio of atoms of each element in a particular compound (for example, glucose: Calculation of the empirical formula from mass data. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — write the empirical formula. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration of a base with an acid. The process of a titration problem follows the same process of any other stoichiometric problem. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some.
From www.tessshebaylo.com
Find Empirical Formula From Combustion Equation Tessshebaylo Titration Empirical Formula a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — a titration is a volumetric technique in which a solution of one reactant (the. Titration Empirical Formula.
From answerfullthemata.z13.web.core.windows.net
Empirical Formula And Molecular Formula Worksheets Titration Empirical Formula — write the empirical formula. The empirical formula of the compound is \(\ce{fe_2o_3}\). The simplest ratio of atoms of each element in a particular compound (for example, glucose: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. At the equivalence point. Titration Empirical Formula.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Empirical Formula a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. The process of a titration problem follows the same process of any other stoichiometric problem. Titration of a base with an acid. At the equivalence point in a neutralization, the moles of acid are. Titration Empirical Formula.
From www.nagwa.com
Lesson Video Empirical and Molecular Formulas Nagwa Titration Empirical Formula Calculation of the empirical formula from mass data. The simplest ratio of atoms of each element in a particular compound (for example, glucose: The process of a titration problem follows the same process of any other stoichiometric problem. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a. Titration Empirical Formula.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Empirical Formula — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Calculation of the empirical formula from mass data. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — write the empirical formula. . Titration Empirical Formula.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration Empirical Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — write the empirical formula. The simplest ratio of atoms of each element in a particular compound (for example, glucose: Titration of a base with an acid. — a titration is a volumetric technique in which a solution of one. Titration Empirical Formula.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration Empirical Formula — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The simplest ratio of atoms of each element in a particular compound (for example, glucose: The process of a titration problem follows the same process of any other stoichiometric problem. At the equivalence. Titration Empirical Formula.
From id.hutomosungkar.com
4+ How To Write Empirical Formulas Trending Hutomo Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). — write the empirical formula. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The process of a titration problem follows the same process of any other stoichiometric problem. a typical titration. Titration Empirical Formula.
From www.chegg.com
Solved Titration to Determine the Empirical Formula of Zinc Titration Empirical Formula The process of a titration problem follows the same process of any other stoichiometric problem. — write the empirical formula. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. At the equivalence point in a neutralization, the moles of acid are equal. Titration Empirical Formula.
From www.natureof3laws.co.in
Empirical Formula & Molecular Formula Chemistry Class 11 Laws Of Nature Titration Empirical Formula The simplest ratio of atoms of each element in a particular compound (for example, glucose: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of. Titration Empirical Formula.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Empirical Formula The process of a titration problem follows the same process of any other stoichiometric problem. Titration of a base with an acid. Calculation of the empirical formula from mass data. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. — write the. Titration Empirical Formula.
From www.numerade.com
A 2.20 g sample of an unknown acid (empirical formula = C3 H4 O3 ) is dissolved in 1.0 L of Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). The simplest ratio of atoms of each element in a particular compound (for example, glucose: — write the empirical formula. Calculation of the empirical formula from mass data. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known. Titration Empirical Formula.
From www.chegg.com
Titration to Determine the Empirical Formula of Zinc Titration Empirical Formula The process of a titration problem follows the same process of any other stoichiometric problem. Titration of a base with an acid. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The empirical formula of the compound is \(\ce{fe_2o_3}\). molecular formulas. Titration Empirical Formula.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Empirical Formula — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration of a base with an acid. The simplest ratio of atoms of each element in a particular compound (for example, glucose: — write the empirical formula. molecular formulas are derived. Titration Empirical Formula.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Empirical Formula a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. — write the empirical formula. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The process of a titration problem follows the same process. Titration Empirical Formula.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Empirical Formula a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. The process of a titration problem follows the same process of any other stoichiometric problem. — write the empirical formula. The empirical formula of the compound is \(\ce{fe_2o_3}\). — a titration is. Titration Empirical Formula.
From www.numerade.com
SOLVED EL EUE PostLaboratory Questions 1 Titration Analysis of Copper Chloride Titration Empirical Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The process of a titration problem follows the same process of any other stoichiometric problem. — write the empirical formula. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing. Titration Empirical Formula.
From www.numerade.com
SOLVEDA 2.20 g sample of an unknown acid (empirical formula .=C3 H4 O3) is dissolved in 1.0 L Titration Empirical Formula — write the empirical formula. Calculation of the empirical formula from mass data. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. At the. Titration Empirical Formula.
From chemistry.stackexchange.com
stoichiometry Empirical formula of Glauber's salt Chemistry Stack Exchange Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The process of a titration problem follows the same process of any other stoichiometric problem. The simplest ratio of atoms of each element in a particular compound (for example, glucose: a typical titration. Titration Empirical Formula.
From www.scribd.com
Activity No. 3 Empirical Formula of A Compound PDF Titration Chemistry Titration Empirical Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — write the empirical formula. The empirical formula of the compound is \(\ce{fe_2o_3}\). The simplest ratio of atoms of each element in a particular compound (for example, glucose: — a titration is a volumetric technique in which a solution of. Titration Empirical Formula.
From www.chegg.com
Titration to Determine the Empirical Formula of Zinc Titration Empirical Formula Titration of a base with an acid. The process of a titration problem follows the same process of any other stoichiometric problem. The simplest ratio of atoms of each element in a particular compound (for example, glucose: — write the empirical formula. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions. Titration Empirical Formula.
From www.scribd.com
Titration Calculations Section A For these questions, your equation will be straight forward to Titration Empirical Formula Calculation of the empirical formula from mass data. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. The simplest ratio of atoms of each element in a particular compound (for example, glucose: The empirical formula of the compound is \(\ce{fe_2o_3}\). molecular formulas. Titration Empirical Formula.
From www.numerade.com
SOLVED Chemistry Question 5) An unknown monoprotic acid has an empirical formula of CqHsOz. The Titration Empirical Formula The simplest ratio of atoms of each element in a particular compound (for example, glucose: Calculation of the empirical formula from mass data. The empirical formula of the compound is \(\ce{fe_2o_3}\). molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — a titration is a volumetric technique in which a. Titration Empirical Formula.
From chemistrypubs.com
Empirical Formula, Molecular Formula Examples, Calculations Chemistrupubs Titration Empirical Formula — write the empirical formula. Titration of a base with an acid. The simplest ratio of atoms of each element in a particular compound (for example, glucose: The process of a titration problem follows the same process of any other stoichiometric problem. The empirical formula of the compound is \(\ce{fe_2o_3}\). — a titration is a volumetric technique in. Titration Empirical Formula.
From www.studocu.com
Stoichiometry Empirical and Molecular Formulas Equilibrium Titration Redox Reactions Studocu Titration Empirical Formula — write the empirical formula. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The empirical formula of the compound is \(\ce{fe_2o_3}\). The process of a titration problem follows the same process of any other stoichiometric problem. At the equivalence point. Titration Empirical Formula.
From www.vrogue.co
Emperical Formula Molecular Formula Examples And Answ vrogue.co Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. — write the empirical formula. The process of a titration problem follows the same process of any other stoichiometric problem. molecular formulas are derived. Titration Empirical Formula.
From www.studocu.com
Lab Report2 titration lab report 1EXPERIMENT 4 A. DATA TABLE FOR EMPIRICAL FORMULA OF Titration Empirical Formula Calculation of the empirical formula from mass data. Titration of a base with an acid. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the.. Titration Empirical Formula.
From www.chegg.com
Titration of Zinc Chloride Trial 1 Trial 2 Trial 3 a. Titration Empirical Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The empirical formula of the compound is \(\ce{fe_2o_3}\). molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — a titration is a volumetric technique in which a solution of one reactant (the. Titration Empirical Formula.
From www.numerade.com
SOLVED Select the two techniques that we learned for Quantitative Chemical Analysis Gravimetric Titration Empirical Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — write the empirical formula. Calculation of the empirical formula from mass data. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. a typical titration analysis involves the use of a. Titration Empirical Formula.
From printablelibmolines.z13.web.core.windows.net
Determining Empirical Formulas Worksheet Key Titration Empirical Formula Titration of a base with an acid. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — write the empirical formula. The process. Titration Empirical Formula.
From www.numerade.com
SOLVEDOne method for determining the purity of aspirin (empirical formula C9 H8 O4 ) is to Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). The process of a titration problem follows the same process of any other stoichiometric problem. — write the empirical formula. Calculation of the empirical formula from mass data. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known. Titration Empirical Formula.
From studylib.net
LABORATORY 4 CONDUCTOMETRIC TITRATIONS Titration Empirical Formula molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. The simplest ratio of atoms of each element in a particular compound (for example, glucose: — write the empirical formula. The empirical formula of the compound is \(\ce{fe_2o_3}\). The process of a titration problem follows the same process of any other. Titration Empirical Formula.
From scienceinfo.com
Empirical Formula Definition, Calculation Titration Empirical Formula a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. The process of a titration problem follows the same process of any other stoichiometric problem. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. Titration. Titration Empirical Formula.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. Titration Empirical Formula The empirical formula of the compound is \(\ce{fe_2o_3}\). Calculation of the empirical formula from mass data. molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. — write the empirical formula. The process of a titration problem follows the same process of any other stoichiometric problem. At the equivalence point in. Titration Empirical Formula.
From www.chegg.com
Solved Titration to Determine the Empirical Formula of Zinc Titration Empirical Formula The simplest ratio of atoms of each element in a particular compound (for example, glucose: Titration of a base with an acid. a typical titration analysis involves the use of a buret (figure 1) to make incremental additions of a solution containing a known concentration of some. — write the empirical formula. The empirical formula of the compound. Titration Empirical Formula.