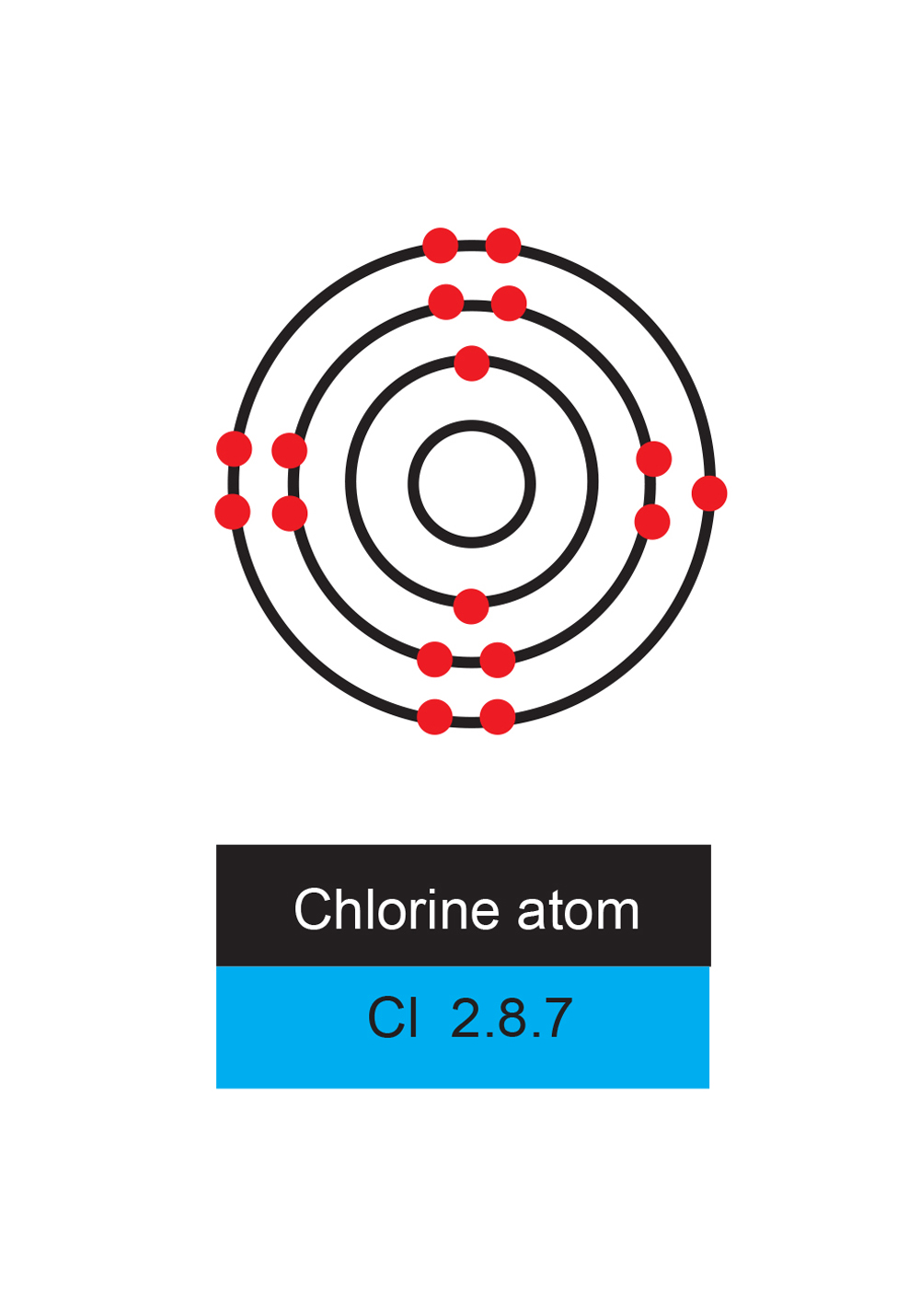
Cl Electron Shells . Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. All of them now have eight electrons, and a filled outer shell! Take a look at the dots around the atoms. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Different shells can hold different maximum numbers of electrons. The atomic number of chlorine is 17. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. This means that the first shell (designated as. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. We'll need to know how many sublevel is present. The electrons in an atom occupy the lowest available energy level first. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form.
from mammothmemory.net
All of them now have eight electrons, and a filled outer shell! When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Different shells can hold different maximum numbers of electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. The electrons in an atom occupy the lowest available energy level first. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. This means that the first shell (designated as.
A certain amount of electrons are allowed in certain shells
Cl Electron Shells We'll need to know how many sublevel is present. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Take a look at the dots around the atoms. We'll need to know how many sublevel is present. All of them now have eight electrons, and a filled outer shell! This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The electrons in an atom occupy the lowest available energy level first. Different shells can hold different maximum numbers of electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. The atomic number of chlorine is 17. This means that the first shell (designated as.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Cl Electron Shells This means that the first shell (designated as. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy. Cl Electron Shells.
From saylordotorg.github.io
Ions Cl Electron Shells The electrons in an atom occupy the lowest available energy level first. Different shells can hold different maximum numbers of electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This means that the first shell (designated as. This implies that chlorine contains a total of 17 protons. Cl Electron Shells.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Cl Electron Shells This means that the first shell (designated as. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. When we write the configuration we'll put all 17 electrons. Cl Electron Shells.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Cl Electron Shells We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of them now have eight electrons, and a filled outer shell! Take a look at the dots around the atoms. This means that the first shell (designated as. When. Cl Electron Shells.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Cl Electron Shells The atomic number of chlorine is 17. The electrons in an atom occupy the lowest available energy level first. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic. Cl Electron Shells.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Cl Electron Shells This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Take a look at the dots around the atoms. The electrons in an atom occupy the lowest available energy level first. Chlorine. Cl Electron Shells.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Cl Electron Shells When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. All of them now have eight electrons,. Cl Electron Shells.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Chlorine Cl Cl Electron Shells When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. This means that the first shell (designated as. All of them now have eight electrons, and a filled outer shell!. Cl Electron Shells.
From utedzz.blogspot.com
Periodic Table Chlorine Bohr Model Periodic Table Timeline Cl Electron Shells We'll need to know how many sublevel is present. The atomic number of chlorine is 17. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This means that the first shell. Cl Electron Shells.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Cl Electron Shells This means that the first shell (designated as. Take a look at the dots around the atoms. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. The electrons in an atom occupy the lowest available energy level first. Chlorine has an atomic number of 17, which means it. Cl Electron Shells.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Cl Electron Shells All of them now have eight electrons, and a filled outer shell! This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Take a look at the dots around the atoms. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This. Cl Electron Shells.
From gzscienceclassonline.weebly.com
1. Electron Configuration Cl Electron Shells Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. This means that the first shell (designated as. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. The electrons in an atom occupy the lowest available energy level first. We'll need. Cl Electron Shells.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Cl Electron Shells This means that the first shell (designated as. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. All of them now have eight electrons, and a filled outer shell! Chlorine has an atomic number of. Cl Electron Shells.
From fionaatbender.blogspot.com
Electron Arrangement of Sodium Chloride FionaatBender Cl Electron Shells The atomic number of chlorine is 17. The electrons in an atom occupy the lowest available energy level first. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. This implies that chlorine contains. Cl Electron Shells.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Cl Electron Shells The atomic number of chlorine is 17. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present. Chlorine (cl) has an atomic number. Cl Electron Shells.
From www.nagwa.com
Lesson Video Electron Shells Nagwa Cl Electron Shells Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Take a look at the dots around the atoms. Different shells can hold different maximum numbers of electrons. The atomic number of chlorine is 17. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in. Cl Electron Shells.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Cl Electron Shells This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. All of them now have eight electrons, and a. Cl Electron Shells.
From www.youtube.com
Chlorine Electron Configuration YouTube Cl Electron Shells Different shells can hold different maximum numbers of electrons. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons. Cl Electron Shells.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Cl Electron Shells All of them now have eight electrons, and a filled outer shell! Take a look at the dots around the atoms. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. The atomic number of chlorine. Cl Electron Shells.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Cl Electron Shells All of them now have eight electrons, and a filled outer shell! This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. We'll need to know how many sublevel is present. The electrons in an atom occupy the lowest available energy level first. Nitrogen shares its electrons with the chlorine atoms, so all. Cl Electron Shells.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Cl Electron Shells The electrons in an atom occupy the lowest available energy level first. We'll need to know how many sublevel is present. All of them now have eight electrons, and a filled outer shell! Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms. Cl Electron Shells.
From sciencenotes.org
Argon Facts Cl Electron Shells Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Different shells can hold different maximum numbers of electrons. We'll need to know how many sublevel is present. Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. When we write the. Cl Electron Shells.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Cl Electron Shells The atomic number of chlorine is 17. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. All of them now have eight electrons, and a filled outer shell! Nitrogen shares its electrons with the chlorine. Cl Electron Shells.
From chemistrytalk.org
Electron Shells ChemTalk Cl Electron Shells We'll need to know how many sublevel is present. This means that the first shell (designated as. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of them now have eight electrons, and a filled outer shell! Different shells can hold different maximum numbers of electrons. When. Cl Electron Shells.
From animalia-life.club
Lewis Dot Structure For Chlorine Cl Electron Shells The atomic number of chlorine is 17. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. We'll need to know how many sublevel is present. This implies that chlorine. Cl Electron Shells.
From www.schoolmykids.com
Compare Chlorine vs Indium Periodic Table Element Comparison Cl Electron Shells All of them now have eight electrons, and a filled outer shell! Different shells can hold different maximum numbers of electrons. This means that the first shell (designated as. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. This implies that chlorine contains a total of 17 protons and 17. Cl Electron Shells.
From animalia-life.club
Potassium Chloride Shell Model Cl Electron Shells Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. The electrons in an atom occupy the lowest available energy level first. Take a look at the dots around the atoms. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with. Cl Electron Shells.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Cl Electron Shells Different shells can hold different maximum numbers of electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. All of them now have eight electrons, and a filled outer shell! We'll need to know how many sublevel is present. Nitrogen shares its electrons with the chlorine atoms, so. Cl Electron Shells.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Cl Electron Shells This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. All of them now have eight electrons, and a. Cl Electron Shells.
From mammothmemory.net
A certain amount of electrons are allowed in certain shells Cl Electron Shells Chlorine (cl) has an atomic number of 17, which means it has 17 electrons distributed in different energy levels or shells. Different shells can hold different maximum numbers of electrons. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The electrons in an atom occupy the lowest available. Cl Electron Shells.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Cl Electron Shells This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. The atomic number of chlorine is 17.. Cl Electron Shells.
From chemtech-us.com
15 Interesting Facts About Chlorine Cl Electron Shells This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the chlorine atom. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. Cl Electron Shells.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Cl Electron Shells This means that the first shell (designated as. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. All. Cl Electron Shells.
From gardenandplate.com
Molecules Cl Electron Shells Different shells can hold different maximum numbers of electrons. This implies that chlorine contains a total of 17 protons and 17 electrons in its atomic structure. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. We'll need to know how many sublevel is present. This means that the first shell (designated as.. Cl Electron Shells.
From 88guru.com
Electron Configuration Rules, Example and Diagram 88Guru Cl Electron Shells Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. The electrons in an atom occupy the lowest available energy level first. All of them now have eight electrons, and a filled outer shell! This means that the first shell (designated as. When we write the configuration we'll put all 17 electrons in. Cl Electron Shells.