Ionic Compound Formed Between Barium And Bromine . The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. The ionic compound formed from barium and bromine is barium bromide,. What is the ionic compound for barium and bromine? If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion.
from www.numerade.com
For example, \(\ce{cabr2}\) contains a metallic element. What is the ionic compound for barium and bromine? If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. Compounds between metal and nonmetal elements are usually ionic. The ionic compound formed from barium and bromine is barium bromide,.
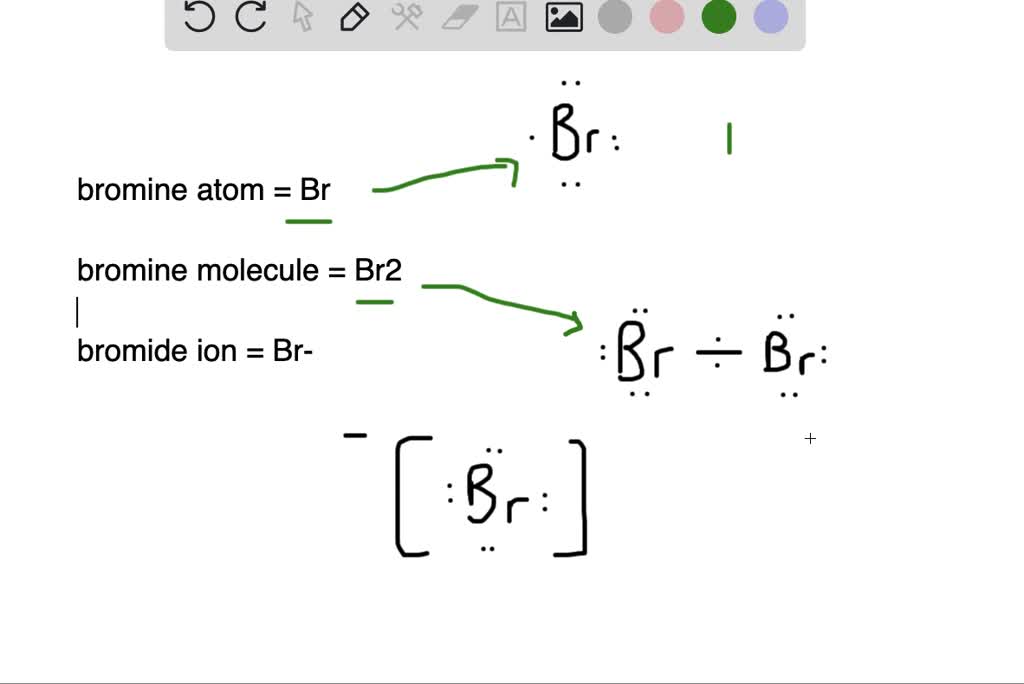
What is the difference between (a) a bromine atom, (b) a bromine
Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. Compounds between metal and nonmetal elements are usually ionic. The ionic compound formed from barium and bromine is barium bromide,. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. What is the ionic compound for barium and bromine?
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. The ionic compound formed from barium and bromine is barium bromide,. For example, \(\ce{cabr2}\) contains a metallic element. Compounds between metal and nonmetal elements are usually ionic. If we look at the ionic compound consisting of. Ionic Compound Formed Between Barium And Bromine.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is. Ionic Compound Formed Between Barium And Bromine.
From www.animalia-life.club
Barium Lewis Dot Structure Ionic Compound Formed Between Barium And Bromine The ionic compound formed from barium and bromine is barium bromide,. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. What is the ionic compound for barium and bromine? Compounds between metal and nonmetal elements are usually ionic. If we look at the ionic compound consisting of lithium ions. Ionic Compound Formed Between Barium And Bromine.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2683450 Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized. Ionic Compound Formed Between Barium And Bromine.
From courses.lumenlearning.com
Lewis Symbols and Structures CHEM 1305 Introductory Chemistry Ionic Compound Formed Between Barium And Bromine Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. For example, \(\ce{cabr2}\) contains a metallic element.. Ionic Compound Formed Between Barium And Bromine.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. What is the ionic compound for barium and bromine? The ionic compound formed from barium and bromine is barium bromide,. For example, \(\ce{cabr2}\) contains a metallic element. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
SOLVED 23 Write a formula for the ionic compound formed from each pair Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. Compounds between metal and nonmetal elements are usually ionic.. Ionic Compound Formed Between Barium And Bromine.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. What is the ionic compound for barium and bromine? Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds). Ionic Compound Formed Between Barium And Bromine.
From brainly.in
Draw an electron dot diagram for barium and bromine and write its Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. Compounds between metal and nonmetal elements are usually ionic. What is the ionic compound for barium and bromine? If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a. Ionic Compound Formed Between Barium And Bromine.
From lambdageeks.com
Barium Lewis Dot Structure Drawing, Several Compounds and Detailed Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions. Ionic Compound Formed Between Barium And Bromine.
From lambdageeks.com
Barium Lewis Dot Structure Drawing, Several Compounds and Detailed Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Compounds between metal and nonmetal elements are usually. Ionic Compound Formed Between Barium And Bromine.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. What is the ionic compound for barium and bromine? The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the. Ionic Compound Formed Between Barium And Bromine.
From www.youtube.com
How to write the equation for Equation for BaBr2 + H2O (Barium bromide Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. The compound formed by this transfer is stabilized. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
SOLVED 1.) The following molecular equation represents the reaction Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. Compounds between metal and nonmetal elements are usually ionic. For example, \(\ce{cabr2}\). Ionic Compound Formed Between Barium And Bromine.
From mungfali.com
Ppt Naming Ionic And Covalent Compounds Powerpoint Presentation, Free A12 Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we. Ionic Compound Formed Between Barium And Bromine.
From stock.adobe.com
Ba Barium Element Information Facts, Properties, Trends, Uses and Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. Compounds between metal and nonmetal elements are usually ionic. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. Ionic Compound Formed Between Barium And Bromine.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Ionic Compound Formed Between Barium And Bromine For example, \(\ce{cabr2}\) contains a metallic element. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. What is the ionic compound for barium and bromine?. Ionic Compound Formed Between Barium And Bromine.
From www.youtube.com
Net ionic equation Ammonium bromide plus barium hydroxide YouTube Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? For example, \(\ce{cabr2}\) contains a metallic element. Compounds between metal and nonmetal elements are usually ionic. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between. Ionic Compound Formed Between Barium And Bromine.
From slideplayer.com
Chemical Bonds The Formation of Compounds From Atoms ppt download Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. The ionic compound formed from barium and. Ionic Compound Formed Between Barium And Bromine.
From slideplayer.com
Ionic Compound Formulas ppt download Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. What is the ionic compound for barium and bromine? If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. For example, \(\ce{cabr2}\). Ionic Compound Formed Between Barium And Bromine.
From sciencenotes.org
Ionic Bond Definition and Examples Ionic Compound Formed Between Barium And Bromine The ionic compound formed from barium and bromine is barium bromide,. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present. Ionic Compound Formed Between Barium And Bromine.
From studylib.net
Ionic Compounds Naming Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. The ionic compound formed from barium and bromine is barium bromide,. For example, \(\ce{cabr2}\) contains a. Ionic Compound Formed Between Barium And Bromine.
From www.bartleby.com
Answered 4. Atoms of the elements barium and… bartleby Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. If we look at the ionic compound consisting of lithium ions and bromide ions, we see. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
SOLVEDWrite a formula for the ionic compound formed from each pair of Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The ionic compound formed from barium and bromine is barium bromide,. The compound formed by this. Ionic Compound Formed Between Barium And Bromine.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Ionic Compound Formed Between Barium And Bromine The ionic compound formed from barium and bromine is barium bromide,. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present. Ionic Compound Formed Between Barium And Bromine.
From ar.inspiredpencil.com
Naming Ionic Compounds Periodic Table Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The ionic compound formed from barium and bromine is barium bromide,. The compound formed by this transfer is stabilized by the electrostatic attractions. Ionic Compound Formed Between Barium And Bromine.
From www.youtube.com
Writing Chemical Formulas For Ionic Compounds YouTube Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this. Ionic Compound Formed Between Barium And Bromine.
From www.showme.com
Ionic Bonding calcium and bromine Science ShowMe Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. What. Ionic Compound Formed Between Barium And Bromine.
From flatworldknowledge.lardbucket.org
Naming Ionic Compounds Ionic Compound Formed Between Barium And Bromine What is the ionic compound for barium and bromine? The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. Compounds between metal and nonmetal elements are usually ionic. For example, \(\ce{cabr2}\) contains a metallic element. The ionic compound formed from barium and bromine is barium bromide,.. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
SOLVED What is the formula of the ionic compound expected to form Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. The ionic compound formed from barium and bromine is barium bromide,. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Compounds. Ionic Compound Formed Between Barium And Bromine.
From courses.lumenlearning.com
3.4 Ionic Nomenclature The Basics of General, Organic, and Biological Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. Compounds between metal and nonmetal elements are usually ionic. What is the ionic compound for barium and bromine? The ionic compound formed from barium and bromine is barium bromide,. The compound formed by this transfer is. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Ionic Compound Formed Between Barium And Bromine If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. The. Ionic Compound Formed Between Barium And Bromine.
From www.numerade.com
SOLVEDPredict the charges of the ions in an ionic compound composed of Ionic Compound Formed Between Barium And Bromine For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. Compounds between metal and nonmetal. Ionic Compound Formed Between Barium And Bromine.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Ionic Compound Formed Between Barium And Bromine The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, \(\ce{cabr2}\) contains a metallic element. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. What. Ionic Compound Formed Between Barium And Bromine.
From www2.victoriacollege.edu
Chapter 2 Atoms, Molecules and Life Chemistry) Ionic Compound Formed Between Barium And Bromine The ionic compound formed from barium and bromine is barium bromide,. For example, \(\ce{cabr2}\) contains a metallic element. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a. Ionic Compound Formed Between Barium And Bromine.