Reference Electrodes Definition Chemistry . The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Here, an experienced team of.
from slidetodoc.com
Here, an experienced team of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to.
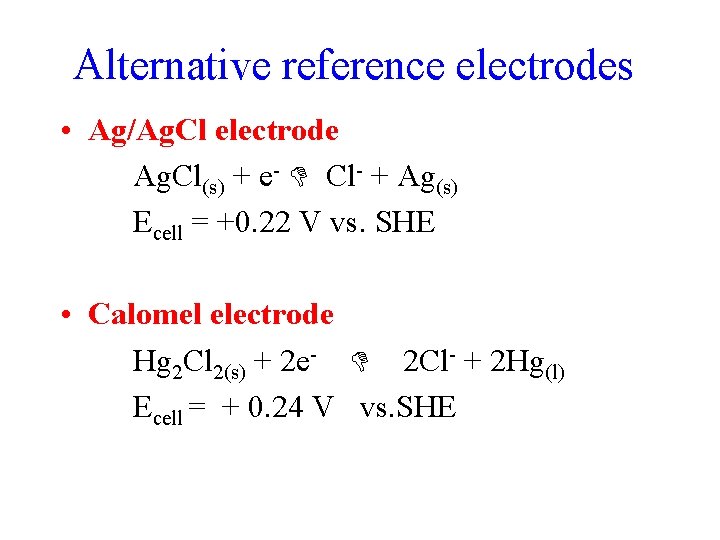
Standard Reference Electrode Standard Hydrogen Electrode SHE SHE
Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Here, an experienced team of.
From www.slideserve.com
PPT Electroanalytical chemistry PowerPoint Presentation, free Reference Electrodes Definition Chemistry Here, an experienced team of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred. Reference Electrodes Definition Chemistry.
From mungfali.com
Types Of Electrodes Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Here, an experienced team of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and measures the potential. Reference Electrodes Definition Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts Reference Electrodes Definition Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. Here, an experienced team of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and measures the potential. Reference Electrodes Definition Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Here, an experienced team of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. Reference electrodes are necessary to control the potential of a working electrode. Reference Electrodes Definition Chemistry.
From gaskatel.de
Reference electrode Gaskatel Reference Electrodes Definition Chemistry A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has. Reference Electrodes Definition Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Reference Electrodes Definition Chemistry Here, an experienced team of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or. Reference Electrodes Definition Chemistry.
From www.youtube.com
Reference electrodes YouTube Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Reference Electrodes Definition Chemistry.
From jenningsanodes.co.uk
Silver/Silver Chloride Probe Reference Electrode Jennings Anodes UK Reference Electrodes Definition Chemistry Here, an experienced team of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and. Reference Electrodes Definition Chemistry.
From www.slideserve.com
PPT Electrochemical Theory PowerPoint Presentation, free download Reference Electrodes Definition Chemistry A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode. Reference Electrodes Definition Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference. Reference Electrodes Definition Chemistry.
From www.researchgate.net
Schematic of the threeelectrode measurement system. Download Reference Electrodes Definition Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode. Reference Electrodes Definition Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Reference Electrodes Definition Chemistry Here, an experienced team of. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential that provides a consistent reference point. Reference Electrodes Definition Chemistry.
From www.slideserve.com
PPT Electroanalytical chemistry PowerPoint Presentation, free Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode. Reference Electrodes Definition Chemistry.
From www.youtube.com
Reference Electrodes YouTube Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. Here, an experienced team of. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference. Reference Electrodes Definition Chemistry.
From www.researchgate.net
Schemes of (a) an allvanadium redox flow battery with a DHE based Reference Electrodes Definition Chemistry A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or. Reference Electrodes Definition Chemistry.
From www.hamiltoncompany.com
The pH Reference Electrode Process Analytics Hamilton Company Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Here, an experienced team of. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements). Reference Electrodes Definition Chemistry.
From pubs.rsc.org
Connecting electrodes with light one wire, many electrodes Chemical Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Here, an experienced team of. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. Reference electrodes are necessary to control the potential of a working electrode. Reference Electrodes Definition Chemistry.
From exoiwzsuc.blob.core.windows.net
What Are The Different Types Of Electrodes at Elenora Spink blog Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Reference Electrodes Definition Chemistry.
From cse.umn.edu
Research on ionic liquidbased reference electrodes "Editors' Choice Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Reference Electrodes Definition Chemistry.
From www.slideserve.com
PPT 141 Reference Electrodes PowerPoint Presentation, free download Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. Here, an experienced. Reference Electrodes Definition Chemistry.
From mungfali.com
Reference Electrode Diagram Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. Here, an experienced team of. A reference electrode is a stable and known electrode potential used as a comparison point. Reference Electrodes Definition Chemistry.
From www.slideserve.com
PPT Electrochemical Theory PowerPoint Presentation, free download Reference Electrodes Definition Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Here, an experienced. Reference Electrodes Definition Chemistry.
From www.slideserve.com
PPT REFERENCE ELECTRODES CHAPTER 4 PowerPoint Presentation, free Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode. Reference Electrodes Definition Chemistry.
From www.thoughtco.com
How to Define Anode and Cathode Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode. Reference Electrodes Definition Chemistry.
From nottingham-repository.worktribe.com
Electrochemical investigation of novel reference electrode Ni/Ni(OH Reference Electrodes Definition Chemistry A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Reference Electrodes Definition Chemistry.
From byjus.com
79.What is reference electrode Reference Electrodes Definition Chemistry The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Reference Electrodes Definition Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode. Reference Electrodes Definition Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts Reference Electrodes Definition Chemistry A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Here, an experienced team of. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential that provides a consistent reference point. Reference Electrodes Definition Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Reference Electrodes Definition Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Here, an experienced. Reference Electrodes Definition Chemistry.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Reference Electrodes Definition Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode. Reference Electrodes Definition Chemistry.
From fr.sdec-france.com
Électrodes de Référence Ag / AgCl pour Mesures de pH et Potentiel Redox Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. Here, an experienced team of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and. Reference Electrodes Definition Chemistry.
From slidetodoc.com
Standard Reference Electrode Standard Hydrogen Electrode SHE SHE Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference. Reference Electrodes Definition Chemistry.
From www.researchgate.net
(PDF) Reference Electrodes Reference Electrodes Definition Chemistry Here, an experienced team of. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode scans the solution close to the metal surface and measures the potential. Reference Electrodes Definition Chemistry.
From cmpgroup.eu
Reference Electrodes CMP Europe Reference Electrodes Definition Chemistry A reference electrode is a stable and known electrode potential that provides a consistent reference point for measuring the voltage of. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or. Reference Electrodes Definition Chemistry.
From studylib.net
Reference Electrodes Reference Electrodes Definition Chemistry Here, an experienced team of. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or. Reference Electrodes Definition Chemistry.