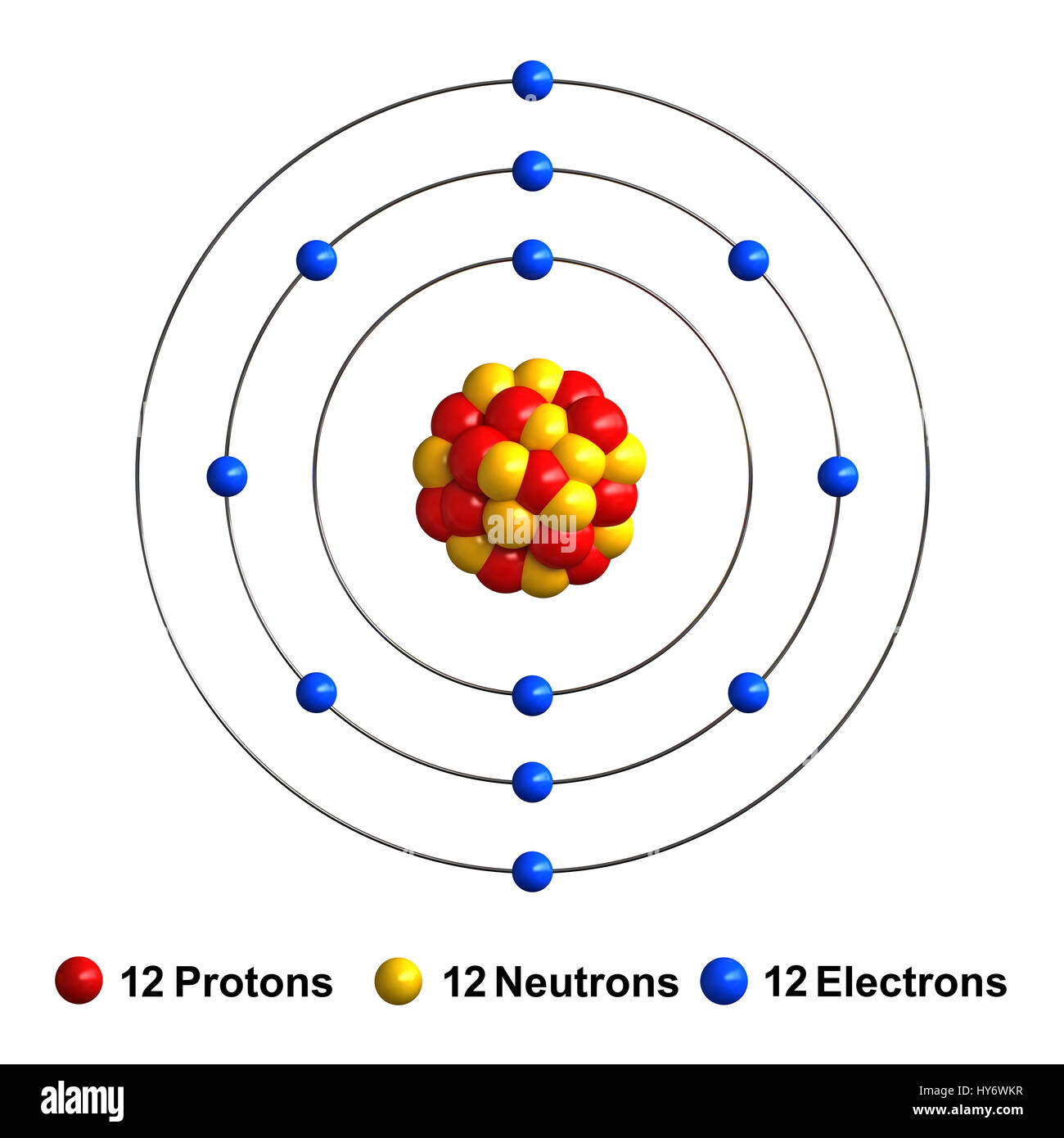
Magnesium Ion Orbital Diagram . Magnesium has two valence electrons in the 3s orbital. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. This diagram shows how the electrons in the. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Since 1s can only hold two electrons. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. To write the orbital diagram for the magnesium atom (mg) first we need to write the.
from www.alamy.com
It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Magnesium has two valence electrons in the 3s orbital. Since 1s can only hold two electrons. To write the orbital diagram for the magnesium atom (mg) first we need to write the. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. This diagram shows how the electrons in the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
3d render of atom structure of magnesium isolated over white background
Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. Since 1s can only hold two electrons. Magnesium has two valence electrons in the 3s orbital. This diagram shows how the electrons in the. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Orbital Diagram The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. Since 1s can only hold two electrons. Magnesium has two valence electrons in the 3s orbital. To write the orbital diagram for the magnesium atom (mg) first we. Magnesium Ion Orbital Diagram.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Ion Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. To write the orbital diagram for the magnesium atom (mg) first we need to write the. This diagram shows how the electrons in the. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded. Magnesium Ion Orbital Diagram.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has two valence electrons in the 3s orbital. Since 1s can only hold two electrons. This diagram shows how the electrons in the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of. Magnesium Ion Orbital Diagram.
From galvinconanstuart.blogspot.com
Magnesium Orbital Diagram General Wiring Diagram Magnesium Ion Orbital Diagram It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals.. Magnesium Ion Orbital Diagram.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Ion Orbital Diagram Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map. Magnesium Ion Orbital Diagram.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Ion Orbital Diagram The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. This diagram shows how the electrons in the. Magnesium has. Magnesium Ion Orbital Diagram.
From diagramacademy.com
Orbital Diagram of Magnesium Magnesium Ion Orbital Diagram The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. To write the orbital diagram for the magnesium atom (mg) first we need to write the.. Magnesium Ion Orbital Diagram.
From wirelistcollegium.z14.web.core.windows.net
Magnesium Orbital Diagram Magnesium Ion Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium refers to the arrangement of electrons in the. Magnesium Ion Orbital Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Orbital Diagram The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. This diagram shows how the electrons in the. In writing the electron configuration for magnesium the first two electrons will go in. Magnesium Ion Orbital Diagram.
From joittsmyb.blob.core.windows.net
Magnesium Lewis Electron Dot Structure at Diane Gilbert blog Magnesium Ion Orbital Diagram Since 1s can only hold two electrons. Magnesium has two valence electrons in the 3s orbital. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. In writing the electron configuration for magnesium the first two electrons will go in the 1s. Magnesium Ion Orbital Diagram.
From guidemanualeruptivity.z14.web.core.windows.net
Magnesium Mg Ground State Orbital Diagram Magnesium Ion Orbital Diagram The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. Magnesium has two valence electrons in. Magnesium Ion Orbital Diagram.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Orbital Diagram The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. Since 1s can only hold two electrons. It describes how electrons are. Magnesium Ion Orbital Diagram.
From www.alamy.com
Magnesium atom diagram concept Stock Vector Image & Art Alamy Magnesium Ion Orbital Diagram Since 1s can only hold two electrons. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The diagram. Magnesium Ion Orbital Diagram.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Ion Orbital Diagram Since 1s can only hold two electrons. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. Magnesium has two valence electrons in the 3s orbital. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. To write the orbital diagram for the magnesium atom (mg) first. Magnesium Ion Orbital Diagram.
From circuitlibbottega.z21.web.core.windows.net
Magnesium Dot Diagram Magnesium Ion Orbital Diagram It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Magnesium has two valence electrons in the 3s orbital. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The electron configuration of magnesium refers to. Magnesium Ion Orbital Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Orbital Diagram The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. In writing the electron configuration for. Magnesium Ion Orbital Diagram.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. This diagram shows how the electrons in the. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides. Magnesium Ion Orbital Diagram.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Ion Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has two valence electrons in the 3s orbital. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is. Magnesium Ion Orbital Diagram.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Ion Orbital Diagram To write the orbital diagram for the magnesium atom (mg) first we need to write the. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. Magnesium has two valence electrons in the 3s orbital. It describes. Magnesium Ion Orbital Diagram.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion Orbital Diagram Since 1s can only hold two electrons. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. This diagram shows how the electrons in the. It describes how electrons are distributed among the various atomic orbitals and energy. Magnesium Ion Orbital Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The diagram of the. Magnesium Ion Orbital Diagram.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C013/1519 Science Photo Library Magnesium Ion Orbital Diagram This diagram shows how the electrons in the. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The diagram of the. Magnesium Ion Orbital Diagram.
From joizkfdrp.blob.core.windows.net
Magnesium Ion Aufbau Diagram at Katherine Cortez blog Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. To write the orbital diagram for the magnesium atom (mg) first we need to write the. This diagram shows how the electrons in the. The magnesium orbital diagram is a graphical representation of the electron configuration. Magnesium Ion Orbital Diagram.
From mungfali.com
Magnesium Orbital Diagram Magnesium Ion Orbital Diagram It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The diagram of the magnesium ion. Magnesium Ion Orbital Diagram.
From nursehub.com
Electron Shells NurseHub Magnesium Ion Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded. Magnesium Ion Orbital Diagram.
From chicfer.blogspot.com
orbital diagram magnesium Chicfer Magnesium Ion Orbital Diagram It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Since 1s can only hold two electrons. To write the orbital diagram for the magnesium atom (mg) first we need to write the. In writing the electron configuration for magnesium the first. Magnesium Ion Orbital Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Orbital Diagram This diagram shows how the electrons in the. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. It describes how electrons are distributed among the various atomic. Magnesium Ion Orbital Diagram.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion Orbital Diagram To write the orbital diagram for the magnesium atom (mg) first we need to write the. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. Magnesium has two valence electrons in the 3s orbital. In writing the electron configuration for magnesium. Magnesium Ion Orbital Diagram.
From schematicfixunshared.z22.web.core.windows.net
Magnesium Orbital Diagram Magnesium Ion Orbital Diagram Since 1s can only hold two electrons. The diagram of the magnesium ion shows a central magnesium atom with a charge of +2, surrounded by a cloud of electrons. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. It describes how electrons are distributed among the various atomic orbitals and energy levels, and. Magnesium Ion Orbital Diagram.
From brainly.com
⚗️What is the electron configuration for the Magnesium ion? Magnesium Ion Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. This diagram shows how the electrons in the. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. The diagram of the magnesium ion shows a central magnesium. Magnesium Ion Orbital Diagram.
From mavink.com
Draw The Orbital Diagram For Magnesium Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. To write the orbital diagram for the magnesium atom (mg) first we need to write the. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It describes how electrons are distributed among the. Magnesium Ion Orbital Diagram.
From quicycle.com
12. Magnesium The Quantum Bicycle Society Magnesium Ion Orbital Diagram In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom. To write the orbital diagram for the magnesium atom (mg) first we need to write the. The. Magnesium Ion Orbital Diagram.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Orbital Diagram Magnesium has two valence electrons in the 3s orbital. Since 1s can only hold two electrons. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. To write the orbital diagram for the magnesium atom (mg) first we need to write the. In writing the electron configuration for magnesium the first two electrons will. Magnesium Ion Orbital Diagram.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Ion Orbital Diagram Magnesium has two valence electrons in the 3s orbital. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium refers to the arrangement of electrons in the magnesium atom’s orbitals. It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed. Magnesium Ion Orbital Diagram.
From uzabytaodi.blogspot.com
41 orbital diagram of magnesium Wiring Diagrams Manual Magnesium Ion Orbital Diagram It describes how electrons are distributed among the various atomic orbitals and energy levels, and provides a detailed map of where each electron is likely to be found. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. To write the orbital diagram for the magnesium atom (mg) first we need to write. Magnesium Ion Orbital Diagram.