Rate Constant K For A Certain Reaction Is 2.3 10^-5 . Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. We know that unit of k. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant.
from www.bartleby.com
The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Where n is the order of reaction. Identify the reaction order from each of the following rate constants. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures:
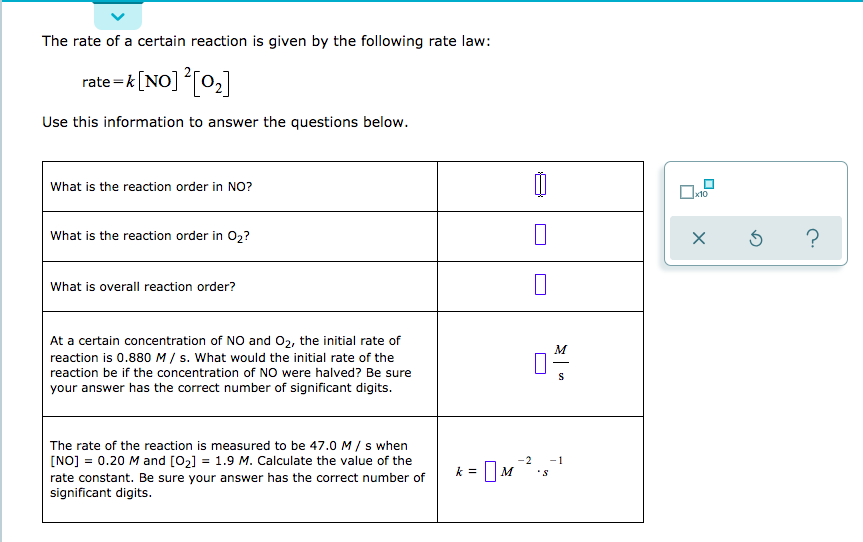
Answered The rate of a certain reaction is given… bartleby
Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. Identify the reaction order from each of the following rate constants. Where n is the order of reaction. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures:
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate Rate Constant K For A Certain Reaction Is 2.3 10^-5 Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. We know that unit of k. The rate constant k for a. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.chegg.com
Solved The rate constant for a certain reaction is k = Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
SOLVED The rate constant for a certain reaction is k = 5.50 Rate Constant K For A Certain Reaction Is 2.3 10^-5 The unit of rate constant. Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: We. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From scoop.eduncle.com
The rate constant of a unimolecular reaction was 2.66 x 10 s and 2.2 x Rate Constant K For A Certain Reaction Is 2.3 10^-5 We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. Identify the reaction order from each of the following rate constants. Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
SOLVED Using the Arrhenius equation to calculate Ea from k versus T Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. Where n is the order of reaction. We know that unit of k. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.toppr.com
Rate constant k for first order reaction has been found to be 2.54 × 10 Rate Constant K For A Certain Reaction Is 2.3 10^-5 We know that unit of k. The unit of rate constant. Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate constant k for a certain reaction is measured at two different temperatures: The rate. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K For A Certain Reaction Is 2.3 10^-5 The unit of rate constant. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: The. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
SOLVED Use the experimental data given in the table and determine the Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. Where n is the order of reaction. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate constant k for a certain reaction is measured at. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
SOLVED The rate constant k for certain reaction is measured at two Rate Constant K For A Certain Reaction Is 2.3 10^-5 The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. Where n. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.chegg.com
Solved The rate constant for a certain reaction is k = Rate Constant K For A Certain Reaction Is 2.3 10^-5 We know that unit of k. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. Where n. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.coursehero.com
[Solved] The rate constant k for a certain reaction is measured at two Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. We. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.toppr.com
The rate constant for the of a certain reaction is Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. The unit of rate constant. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate constant k for a. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From oneclass.com
OneClass The rate constant k for a certain reaction is measured at two Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: The rate constant k for a. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate constant. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.bartleby.com
Answered The rate of a certain reaction is given… bartleby Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
SOLVEDConsider the following reaction, where the rate constant k Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. We know that unit of k. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate constant. Identify the reaction order from each. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.numerade.com
The rate constant (k) for a reaction is measured as a function of Rate Constant K For A Certain Reaction Is 2.3 10^-5 Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. The rate. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From kunduz.com
[ANSWERED] The rate constant k for a certain reaction is measured at Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. Where n is the order of reaction. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Where n is the order of reaction. The unit of rate constant. We know that unit of k. The rate constant k for a certain reaction is. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.slideshare.net
Chapter 14 Lecture Chemical Rate Constant K For A Certain Reaction Is 2.3 10^-5 The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Rate Constant K For A Certain Reaction Is 2.3 10^-5 Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. Where n is the order of reaction. Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From answerhappy.com
The rate of a certain reaction was studied at various temperatures. The Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. Where n is the order of reaction. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.toppr.com
The rate constant (K) for the reaction 2A + B→ product, was found to be Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. The unit of rate constant. Rate constant of. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From askfilo.com
Rate constant k for a first order reaction has been found to be 2.54×10−3.. Rate Constant K For A Certain Reaction Is 2.3 10^-5 We know that unit of k. The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.researchgate.net
data, including second order rate constants (k 2 ), for the Rate Constant K For A Certain Reaction Is 2.3 10^-5 The unit of rate constant. The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: Rate constant of first order reaction is the constant of proportionality to initial concentration and the. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant K For A Certain Reaction Is 2.3 10^-5 Where n is the order of reaction. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Identify the reaction order from each of the following rate constants. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. We. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant K For A Certain Reaction Is 2.3 10^-5 Identify the reaction order from each of the following rate constants. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. Where n is the order of reaction. We know that unit of k. The rate constant k for a certain reaction is measured at two different temperatures:. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From www.toppr.com
The rate constant of a certain reaction is given by log10K = 5.3192 Rate Constant K For A Certain Reaction Is 2.3 10^-5 Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant reacted or. The rate constant k for a certain reaction is measured at two different temperatures: The rate constant k for a certain reaction is measured at two different temperatures: Identify the reaction order from each of the following rate. Rate Constant K For A Certain Reaction Is 2.3 10^-5.
From byjus.com
a first ordre rection has a rate constant of 2.303 into 10 power minus Rate Constant K For A Certain Reaction Is 2.3 10^-5 The rate constant k for a certain reaction is measured at two different temperatures: Where n is the order of reaction. The rate constant k for a certain reaction is measured at two different temperatures: The unit of rate constant. Rate constant of first order reaction is the constant of proportionality to initial concentration and the amount of the reactant. Rate Constant K For A Certain Reaction Is 2.3 10^-5.