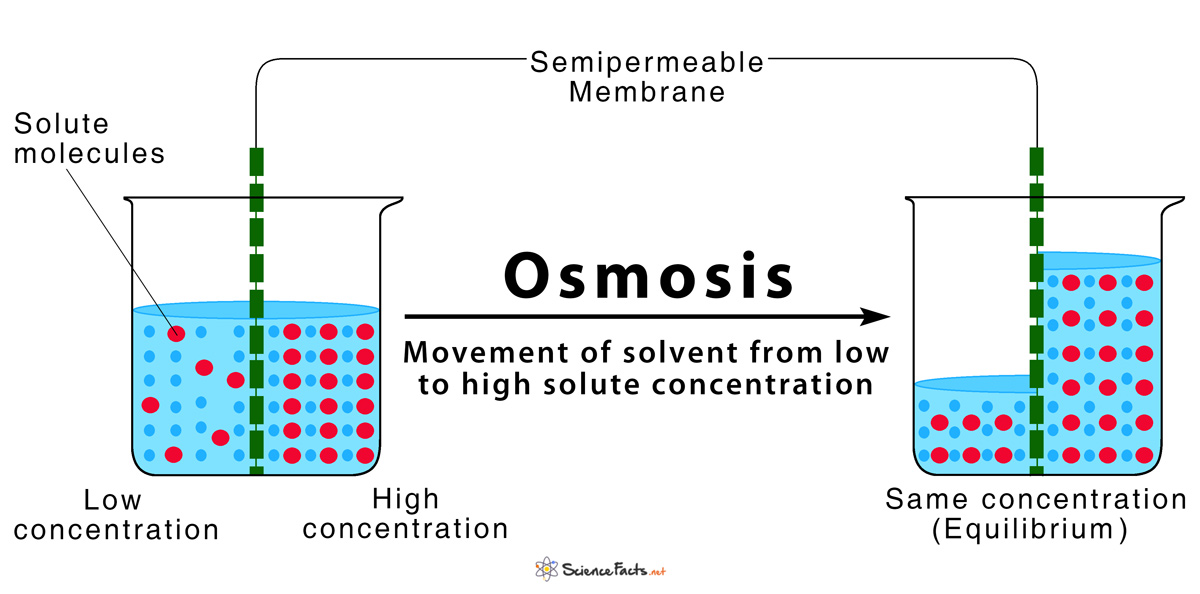
How Do You Make Reverse Osmosis Water . In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. *note that sodium is a small ion, so ro systems. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a.
from www.congress-intercultural.eu
Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. *note that sodium is a small ion, so ro systems. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a.
What Is Osmosis Process Hypertonic, Hypotonic And Isotonic, 45 OFF
How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. *note that sodium is a small ion, so ro systems. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration.
From www.water-rightgroup.com
How Do Reverse Osmosis Systems Work? WaterRight How Do You Make Reverse Osmosis Water Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis, water is. How Do You Make Reverse Osmosis Water.
From www.biopure.com.au
How Reverse Osmosis Works Biopure Water For Life How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes. How Do You Make Reverse Osmosis Water.
From medium.com
Stokk Reverse Osmosis System Review Pure Water Delight by Daniel How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. *note that sodium is a small ion, so ro systems. Reverse osmosis occurs when. How Do You Make Reverse Osmosis Water.
From aquascienceaz.com
Water Solutions from Aqua Science How Do You Make Reverse Osmosis Water Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Some bacteria, fungi, algae, protozoa, and viruses. *note that sodium is a. How Do You Make Reverse Osmosis Water.
From purewatersystems.com.au
What is Reverse Osmosis Water Purification? & Pure Water Systems How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis is the reversal of this. How Do You Make Reverse Osmosis Water.
From jugfree.com
Simple Ways to Make Reverse Osmosis Water Alkaline Jug Free How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. *note that sodium is a small ion, so ro systems. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Some. How Do You Make Reverse Osmosis Water.
From medium.com
The Cross Flow Filtration Of Reverse Osmosis System by O’Brien Water How Do You Make Reverse Osmosis Water Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis occurs when the water. How Do You Make Reverse Osmosis Water.
From www.walmart.com
Yongwei Automatic Shut off Valve Water Purifier RO Reverse Osmosis How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis occurs when the water is moved across the membrane against the concentration. How Do You Make Reverse Osmosis Water.
From joijbeczq.blob.core.windows.net
Does Reverse Osmosis Water Contain Salt at Willa Perkins blog How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse. How Do You Make Reverse Osmosis Water.
From purewaterblog.com
Is Reverse Osmosis Water Soft Water? Water Treatment How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. A reverse osmosis system removes sediment and chlorine from. How Do You Make Reverse Osmosis Water.
From qualitywaterpro.com
Is Reverse Osmosis Water Alkaline? Quality Water Pro How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. In reverse osmosis, water is passed through a selectively permeable membrane from. How Do You Make Reverse Osmosis Water.
From netsolwater.com
Why reverse osmosis has a water rescuing or saving technology How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Some bacteria, fungi, algae, protozoa, and viruses. *note that sodium is a small ion, so ro systems. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis. How Do You Make Reverse Osmosis Water.
From dashappliances.com
How to Make Reverse Osmosis Water Less Acidic? How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Some bacteria, fungi, algae, protozoa, and viruses. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. *note that sodium is a small ion,. How Do You Make Reverse Osmosis Water.
From netsolwater.com
Why is Reverse Osmosis water the healthiest How Do You Make Reverse Osmosis Water Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. *note that sodium is a. How Do You Make Reverse Osmosis Water.
From dashappliances.com
How to Make Reverse Osmosis Water Less Acidic? How Do You Make Reverse Osmosis Water Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. *note that sodium is a small ion,. How Do You Make Reverse Osmosis Water.
From customwater.com
Reverse Osmosis Bottled Water How its Made How Do You Make Reverse Osmosis Water Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis is the reversal of. How Do You Make Reverse Osmosis Water.
From www.aquaprofessor.com
Does Reverse Osmosis Water Dehydrate You? (Hint No, But You'll How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. *note that sodium is a small ion, so ro systems. Some bacteria, fungi, algae, protozoa, and viruses. In reverse. How Do You Make Reverse Osmosis Water.
From www.walmart.com
TUMALL RO Water Faucet Reverse Osmosis Purifier Filtration Drinking How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis occurs when the water is. How Do You Make Reverse Osmosis Water.
From joiwgsgrw.blob.core.windows.net
Can I Use Reverse Osmosis Water For Neti Pot at Joann Randolph blog How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a. How Do You Make Reverse Osmosis Water.
From www.walmart.com
TUMALL RO Water Faucet Reverse Osmosis Purifier Filtration Drinking How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. In reverse. How Do You Make Reverse Osmosis Water.
From www.youtube.com
223 How to Make Reverse Osmosis Water from a Cold Water Line DIY How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Reverse osmosis is the reversal of this natural phenomenon, by the application. How Do You Make Reverse Osmosis Water.
From www.pinterest.com
Stages in Reverse Osmosis Visual.ly in 2020 Reverse osmosis water How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. *note that sodium is a small ion, so ro systems. A reverse osmosis system. How Do You Make Reverse Osmosis Water.
From www.guardianwaterservices.com
Reverse Osmosis & Drinking Water Systems Guardian How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. In reverse osmosis, water is passed through a selectively permeable membrane from a. How Do You Make Reverse Osmosis Water.
From www.homewaterfiltration.com.au
Reverse Osmosis Home Water Filtration How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis,. How Do You Make Reverse Osmosis Water.
From purewaterblog.com
Why Is My Reverse Osmosis Water Green? Water Treatment How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. *note that sodium is a small ion, so ro systems. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. In reverse osmosis, water is passed through a selectively. How Do You Make Reverse Osmosis Water.
From blog.thedistilledwatercompany.com
Reverse Osmosis Water Buy the Highest Quality How Do You Make Reverse Osmosis Water Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to. How Do You Make Reverse Osmosis Water.
From waterontop.com
Reverse Osmosis Vs Alkaline Water (2023 Comparison) How Do You Make Reverse Osmosis Water In reverse osmosis, water is passed through a selectively permeable membrane from a region of high solute concentration to a region of low solute concentration through. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis occurs when the water is. How Do You Make Reverse Osmosis Water.
From colemanhanna.com
Water Treatment Solutions Coleman Hanna Carwash Systems How Do You Make Reverse Osmosis Water Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. A reverse osmosis system. How Do You Make Reverse Osmosis Water.
From www.walmart.com
TUMALL RO Water Faucet Reverse Osmosis Purifier Filtration Drinking How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the. How Do You Make Reverse Osmosis Water.
From www.congress-intercultural.eu
What Is Osmosis Process Hypertonic, Hypotonic And Isotonic, 45 OFF How Do You Make Reverse Osmosis Water *note that sodium is a small ion, so ro systems. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Reverse osmosis occurs when the water is moved across the. How Do You Make Reverse Osmosis Water.
From alkalinewatermachinesource.com
How to Safely Make Reverse Osmosis Water Alkaline at Home How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. Some bacteria, fungi, algae, protozoa, and viruses. In reverse osmosis, water is passed through a selectively permeable membrane from a. How Do You Make Reverse Osmosis Water.
From klaqxcyin.blob.core.windows.net
Can You Use Reverse Osmosis Water Instead Of Distilled Water at Gordon How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. *note that sodium is a small ion, so ro systems. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. In reverse osmosis, water is passed through a selectively. How Do You Make Reverse Osmosis Water.
From purewaterblog.com
Why Is My Reverse Osmosis Water pH High? Water Treatment How Do You Make Reverse Osmosis Water Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. Some bacteria, fungi, algae, protozoa, and viruses. *note that sodium is a small ion, so ro systems. Reverse osmosis takes place when pressure applied to a highly concentrated solute solution causes the solvent to pass through a. In reverse. How Do You Make Reverse Osmosis Water.
From www.water-rightgroup.com
How Do Reverse Osmosis Systems Work? WaterRight How Do You Make Reverse Osmosis Water A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. *note that sodium is a small ion, so ro systems. Reverse osmosis is the reversal of this natural phenomenon,. How Do You Make Reverse Osmosis Water.
From www.walmart.com
Yongwei Automatic Shut off Valve Water Purifier RO Reverse Osmosis How Do You Make Reverse Osmosis Water Reverse osmosis is the reversal of this natural phenomenon, by the application of external pressure on the solution that contains the higher concentration of dissolved ions, this forces. A reverse osmosis system removes sediment and chlorine from water with a prefilter before it forces water through a semipermeable. Some bacteria, fungi, algae, protozoa, and viruses. Reverse osmosis occurs when the. How Do You Make Reverse Osmosis Water.