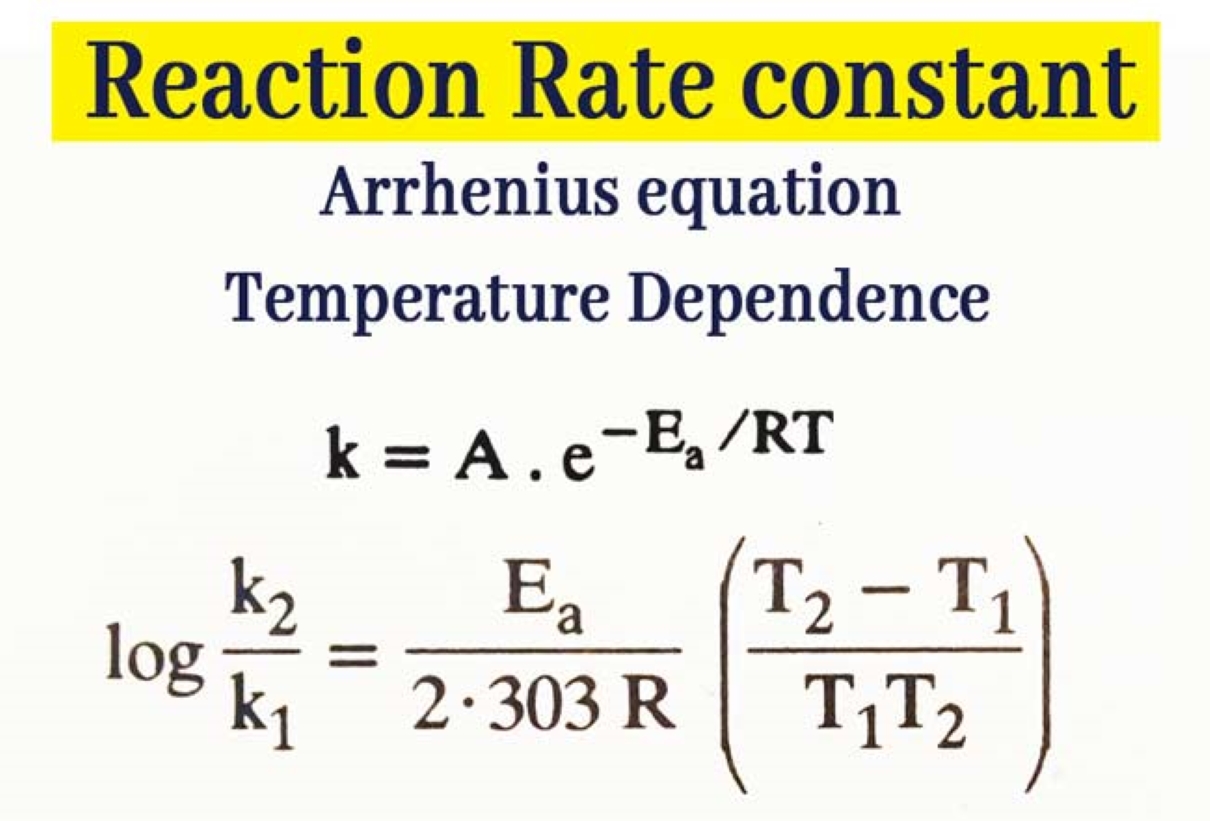
Rate Constant Definition Class 12 . Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • derive integrated rate equations. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Rate constant can be calculated by using the arrhenius equation or by using the molar.
from facts.net
The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • derive integrated rate equations. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst;
13 Mindblowing Facts About Reaction Rate Constant
Rate Constant Definition Class 12 Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Rate constant can be calculated by using the arrhenius equation or by using the molar. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; • derive integrated rate equations. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation ID5609537 Rate Constant Definition Class 12 • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Rate constant can be calculated by using the arrhenius equation or by using the molar. • derive integrated rate equations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how. Rate Constant Definition Class 12.
From www.youtube.com
Rate constant in chemical Rate constant definition define Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. The rate constant is denoted. Rate Constant Definition Class 12.
From www.meritnation.com
Define rate law rate constant Explain rate law with two example Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to. Rate Constant Definition Class 12.
From www.youtube.com
Class12 (CHEMISTRY) CHAPTERCHEMICAL , TOPICUNIT OF RATE Rate Constant Definition Class 12 Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to write and use rate. Rate Constant Definition Class 12.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant Definition Class 12 • discuss the dependence of rate of reactions on concentration, temperature and catalyst; • derive integrated rate equations. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant is denoted by k. Rate Constant Definition Class 12.
From www.youtube.com
Rate Law and Rate constant Chemical Class 12 NCERT Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Rate constant can be calculated by using the arrhenius equation or by using the molar. • derive integrated rate equations. Learn what rate constant is, how. Rate Constant Definition Class 12.
From www.youtube.com
Reaction Rate vs Rate Constant Quick differences and comparison Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to determine the rate law and the specific rate constant. Rate Constant Definition Class 12.
From classnotes.org.in
Integrated Rate Expression Chemical Chemistry, Class 12 Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • derive integrated rate equations. Learn how to write and use rate laws to describe the relationship between. Rate Constant Definition Class 12.
From www.youtube.com
Units of rate constant Chemical Class 12 Boards Neet Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • derive integrated rate equations. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to determine the rate law and the. Rate Constant Definition Class 12.
From gamesmartz.com
Specific Rate Constant Definition & Image GameSmartz Rate Constant Definition Class 12 • derive integrated rate equations. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Rate constant can be calculated by using the arrhenius equation or by. Rate Constant Definition Class 12.
From byjus.com
a first ordre rection has a rate constant of 2.303 into 10 power minus Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • derive integrated rate equations. Rate constant can be calculated by using the arrhenius equation or by using the molar. • discuss the dependence of. Rate Constant Definition Class 12.
From www.youtube.com
9) Units of Rate constant How to calculate units of Rate constant Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn how to write and use rate laws to describe the. Rate Constant Definition Class 12.
From www.iitianacademy.com
NEET Chemistry Chemical Study Notes IBDP,MYP,AP,DSAT.. Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn what rate constant is, how it relates to the. Rate Constant Definition Class 12.
From mavink.com
Rate Constant Calculation Rate Constant Definition Class 12 Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Rate constant can be calculated. Rate Constant Definition Class 12.
From www.youtube.com
Rate law or Rate expression or Rate equation (chemical part 17 Rate Constant Definition Class 12 Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • discuss. Rate Constant Definition Class 12.
From askfilo.com
The rate constant for a first order reaction is 20 min−1. The time requir.. Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Rate constant can be calculated by using the arrhenius equation or by using the molar. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; • derive integrated rate equations. Learn what rate constant is, how it relates to the. Rate Constant Definition Class 12.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. • derive integrated rate equations. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn how to determine the rate law and the. Rate Constant Definition Class 12.
From www.youtube.com
TRICK TO FIND UNIT OF RATE CONSTANT,ORDER OF REACTIONS, XII YouTube Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Learn what rate constant is, how it relates to the molar. Rate Constant Definition Class 12.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant Definition Class 12 Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant is. Rate Constant Definition Class 12.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Reaction Tessshebaylo Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • discuss. Rate Constant Definition Class 12.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Rate Constant Definition Class 12 Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. Rate constant can be calculated by using the arrhenius equation or by using the molar. The rate constant is denoted by k and known as. Rate Constant Definition Class 12.
From www.scribd.com
Class XII Chemistry Ch. 4 Chemical Chapter Notes Key Rate Constant Definition Class 12 • derive integrated rate equations. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn what rate constant is, how it relates to the. Rate Constant Definition Class 12.
From www.doubtnut.com
Explain the difference between instantaneous rate of a reaction and Rate Constant Definition Class 12 • derive integrated rate equations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • discuss the dependence of rate of reactions on concentration, temperature and. Rate Constant Definition Class 12.
From www.meritnation.com
Define half life of a reaction Derive the relationship between half Rate Constant Definition Class 12 • discuss the dependence of rate of reactions on concentration, temperature and catalyst; • derive integrated rate equations. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn how to determine the rate law and the. Rate Constant Definition Class 12.
From facts.net
14 Fascinating Facts About Rate Constant Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. • derive integrated. Rate Constant Definition Class 12.
From www.youtube.com
calculate rate constant of a reaction at 318k 26Unit 4chemical Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. •. Rate Constant Definition Class 12.
From www.youtube.com
Rate Constant And Its Units 💥 Chemical Class 12 Chemistry Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. • derive integrated rate equations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to write and use rate laws to describe the relationship between reaction rate. Rate Constant Definition Class 12.
From www.meritnation.com
The rate constant of the production of 2B(g) by the reaction, Ag →∆2Bg Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to determine the rate law and the specific rate constant. Rate Constant Definition Class 12.
From www.doubtnut.com
According to Arrhenius equation rate constant k is equal to Ae^(E(a)/ Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn what rate constant is, how it relates to the molar concentration. Rate Constant Definition Class 12.
From schools.aglasem.com
CBSE Class 12 Chemistry Notes Chemical AglaSem Schools Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature.. Rate Constant Definition Class 12.
From www.youtube.com
Unit of Rate Constant of First Order & Zero Order Reaction Chemical Rate Constant Definition Class 12 Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. The rate constant is denoted. Rate Constant Definition Class 12.
From www.youtube.com
unit of rate constant chemical class 12 Neet YouTube Rate Constant Definition Class 12 The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. • derive integrated rate equations. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to write and use rate laws to describe the relationship between. Rate Constant Definition Class 12.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn what rate constant is, how it relates to the molar concentration of reactants and the rate of reaction, and how it depends on temperature. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. The rate constant. Rate Constant Definition Class 12.
From www.slideserve.com
PPT Reaction Rate Law PowerPoint Presentation, free download ID5734404 Rate Constant Definition Class 12 Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to determine the rate law and the specific rate constant for a chemical reaction experimentally. • discuss the dependence of rate of reactions on concentration, temperature and catalyst; Learn how to write and use rate laws to describe the relationship between reaction rate. Rate Constant Definition Class 12.
From facts.net
13 Mindblowing Facts About Reaction Rate Constant Rate Constant Definition Class 12 • discuss the dependence of rate of reactions on concentration, temperature and catalyst; • derive integrated rate equations. The rate constant is denoted by k and known as reaction rate constant or specific reaction rate. Rate constant can be calculated by using the arrhenius equation or by using the molar. Learn how to determine the rate law and the specific. Rate Constant Definition Class 12.