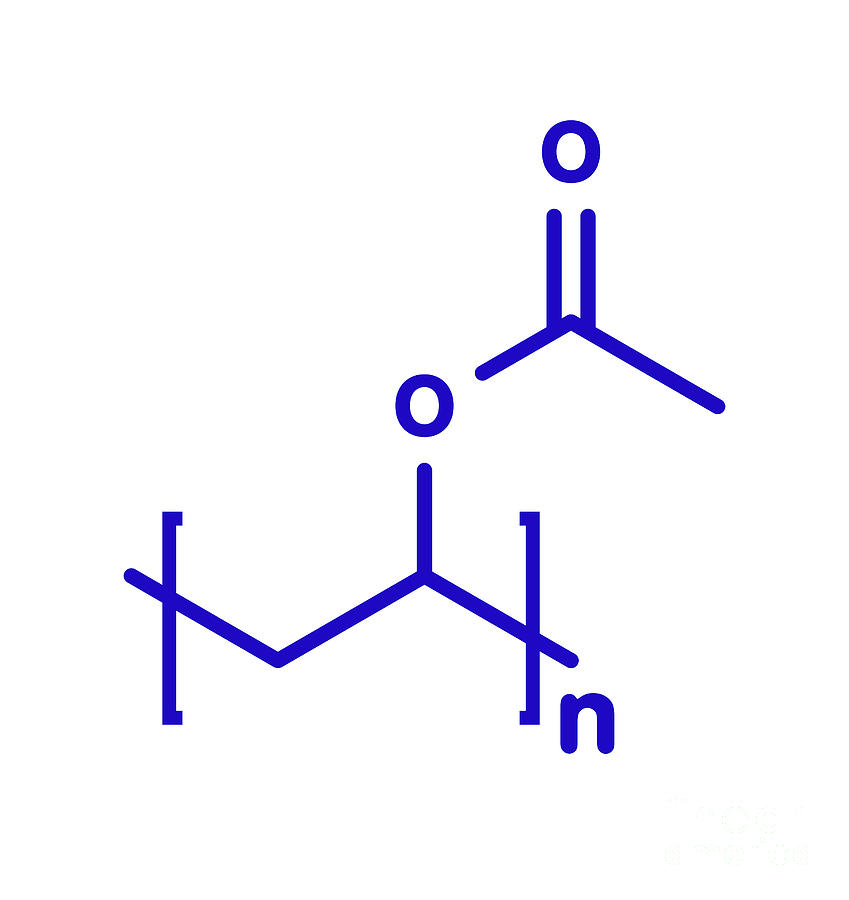
Polyvinyl Acetate In Ethanol . Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. It has the idealized formula [ch 2 ch(oh)] n. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic.
from sciencephotogallery.com
Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Pva is used as an emulsion. Polyvinyl acetate is hydrolyzed into pva [4]. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. It has the idealized formula [ch 2 ch(oh)] n.
Polyvinyl Acetate Polymer Chemical Structure 3 by Molekuul/science
Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into pva [4]. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers.
From www.researchgate.net
FTIR spectrum of polyvinyl acetate (PVAc) polymer Download Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate is hydrolyzed into pva [4]. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a. Polyvinyl Acetate In Ethanol.
From mugeek.vidalondon.net
Chemical Makeup Of Alcohol Mugeek Vidalondon Polyvinyl Acetate In Ethanol The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. It has the idealized formula [ch 2 ch(oh)] n. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples. Polyvinyl Acetate In Ethanol.
From www.numerade.com
SOLVED (A) The polymer known as polyvinyl acetate (PVAc) is used in Polyvinyl Acetate In Ethanol When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Pva is used as an emulsion. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. The solubility of the commercial food grade pva with. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Proposed ion induced main degradation reactions of polyvinyl polymers Polyvinyl Acetate In Ethanol Pva is used as an emulsion. Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. It has. Polyvinyl Acetate In Ethanol.
From einvoice.fpt.com.vn
Characterization Of Alkali Catalyzed Synthesis Of Polyvinyl, 54 OFF Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Pva is used as an emulsion. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh). Polyvinyl Acetate In Ethanol.
From studylib.net
doc Polyvinyl Acetate In Ethanol It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate is hydrolyzed into pva [4]. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. When polyvinyl acetate is alcoholized. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Synthesis and characterization of starch stabilized polyvinyl acetate Polyvinyl Acetate In Ethanol When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Pva is used as an emulsion. Polyvinyl acetate is hydrolyzed into pva [4]. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly,. Polyvinyl Acetate In Ethanol.
From www.plastmagazine.it
Alcool polivinilico (PVA, PVOH, PVAl) che cos’è e a cosa serve il PVA Polyvinyl Acetate In Ethanol The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Pva is used as an emulsion. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. It has. Polyvinyl Acetate In Ethanol.
From www.polysciences.com
Poly(vinyl acetate) Polysciences, Inc. Polyvinyl Acetate In Ethanol When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into pva [4]. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis. Polyvinyl Acetate In Ethanol.
From exydmchoz.blob.core.windows.net
Ethylene And Vinyl Acetate Synthesis at Kathryn Scott blog Polyvinyl Acetate In Ethanol When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate serves as the precursor for polyvinyl alcohol. Polyvinyl Acetate In Ethanol.
From polymersinchemistry.blogspot.com
Poly ( vinyl acetate). Polyvinyl Acetate In Ethanol The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Pva is used as an emulsion. Polyvinyl acetate. Polyvinyl Acetate In Ethanol.
From www.vectorstock.com
Polyvinyl acetate polymer chemical structure Vector Image Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate serves as the precursor for polyvinyl alcohol. Polyvinyl Acetate In Ethanol.
From www.mdpi.com
Polymers Free FullText Cellulosic/Polyvinyl Alcohol Composite Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate is hydrolyzed into pva [4]. The solubility. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Repeating units of poly(vinyl acetate), poly(vinyl alcohol) and Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Mechanism of stabilized polyvinyl acetate (PVAc) using starch Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Pva is used as an emulsion. The solubility of the commercial food. Polyvinyl Acetate In Ethanol.
From www.easthony.com
Polyvinyl Alcohol&Polyvinyl Acetate Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate is hydrolyzed into pva. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Scheme representing the structure of (i) polyvinyl formal (PVF), (ii Polyvinyl Acetate In Ethanol Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate is hydrolyzed into pva [4]. It has the idealized formula. Polyvinyl Acetate In Ethanol.
From joiivjrkv.blob.core.windows.net
How To Dissolve Polyvinyl Alcohol at Alice Foster blog Polyvinyl Acetate In Ethanol Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into. Polyvinyl Acetate In Ethanol.
From tangzhicellulose.en.made-in-china.com
Polyvinyl Alcohol PVA for Polyvinyl Acetate Emulsion Tile Adhesive with Polyvinyl Acetate In Ethanol It has the idealized formula [ch 2 ch(oh)] n. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples. Polyvinyl Acetate In Ethanol.
From www.mdpi.com
Symmetry Free FullText Porous Polyvinyl Alcohol Membranes Polyvinyl Acetate In Ethanol Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food. Polyvinyl Acetate In Ethanol.
From www.youtube.com
Poly(vinyl alcohol) PVA Some important Polymers UG PaathShaala Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Pva is used as an emulsion. The solubility. Polyvinyl Acetate In Ethanol.
From www.differencebetween.com
What is the Difference Between Polyvinyl Alcohol and Polyvinyl Acetate Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into pva [4]. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers.. Polyvinyl Acetate In Ethanol.
From brunofuga.adv.br
Polyvinyl Alcohol (PVA) Applications And Specifications, 43 OFF Polyvinyl Acetate In Ethanol Pva is used as an emulsion. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate is hydrolyzed into pva [4]. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity. Polyvinyl Acetate In Ethanol.
From www.alamy.com
Polyvinyl acetate (PVA) polymer and vinyl acetate monomer molecule Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Pva is used as an emulsion. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5. Polyvinyl Acetate In Ethanol.
From pixels.com
Polyvinyl Acetate Polymer Chemical Structure Photograph by Molekuul Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Pva is used as an. Polyvinyl Acetate In Ethanol.
From polymersinchemistry.blogspot.com
Polyvinyl alcohol. Polyvinyl Acetate In Ethanol The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl. Polyvinyl Acetate In Ethanol.
From sciencephotogallery.com
Polyvinyl Acetate Polymer Chemical Structure 3 by Molekuul/science Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into pva [4]. Pva is used as an emulsion. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. When polyvinyl acetate is alcoholized with sodium. Polyvinyl Acetate In Ethanol.
From www.mdpi.com
IJERPH Free FullText Degradation of Polyvinyl Alcohol in US Polyvinyl Acetate In Ethanol Polyvinyl acetate is hydrolyzed into pva [4]. Pva is used as an emulsion. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. It has the idealized formula. Polyvinyl Acetate In Ethanol.
From scienceprog.com
The 5 Applications Of Polyvinyl Acetate (PVA) Do It Easy With ScienceProg Polyvinyl Acetate In Ethanol It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Polyvinyl acetate is hydrolyzed into pva [4]. Pva is used as an emulsion. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Polyvinyl alcohol stabilized polyvinyl acetate emulsion Download Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8 to. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a. Polyvinyl Acetate In Ethanol.
From www.researchgate.net
Mechanism of stabilized polyvinyl acetate (PVAc) using starch Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Polyvinyl acetate is hydrolyzed into pva [4]. It has the idealized formula [ch 2 ch(oh)] n. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. Pva is. Polyvinyl Acetate In Ethanol.
From www.scribd.com
Polyvinyl acetate _Polyvinyl alcohol PDF Polyvinyl Chloride Ethanol Polyvinyl Acetate In Ethanol When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. It has the idealized formula [ch 2 ch(oh)] n. Polyvinyl acetate is hydrolyzed into pva [4]. Pva is used as an emulsion. The solubility of the commercial food grade pva with certification regarding the degree. Polyvinyl Acetate In Ethanol.
From www.mdpi.com
Foods Free FullText Determination of Polyvinyl Acetate in Chewing Polyvinyl Acetate In Ethanol Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are two commonly used polymers with distinct properties and applications. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. It has the idealized formula [ch 2 ch(oh)] n. The solubility of the commercial food. Polyvinyl Acetate In Ethanol.
From fineartamerica.com
Polyvinyl Acetate Polymer Chemical Structure Photograph by Molekuul Polyvinyl Acetate In Ethanol Polyvinyl acetate serves as the precursor for polyvinyl alcohol and, directly or indirectly, the polyvinyl acetals. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. Pva is used as an emulsion. Polyvinyl acetate is hydrolyzed into pva [4]. The solubility of the commercial food. Polyvinyl Acetate In Ethanol.
From eureka-patsnap-com.libproxy.mit.edu
Alcoholysis method of polyvinyl acetate Eureka Patsnap develop Polyvinyl Acetate In Ethanol Polyvinyl acetate (pva) and polyvinyl alcohol (pvoh) are further examples of ethylene copolymers. When polyvinyl acetate is alcoholized with sodium hydroxide (naoh) as a basic. Pva is used as an emulsion. Polyvinyl acetate is hydrolyzed into pva [4]. The solubility of the commercial food grade pva with certification regarding the degree of hydrolysis (86.5 to 89 %) and viscosity (4.8. Polyvinyl Acetate In Ethanol.