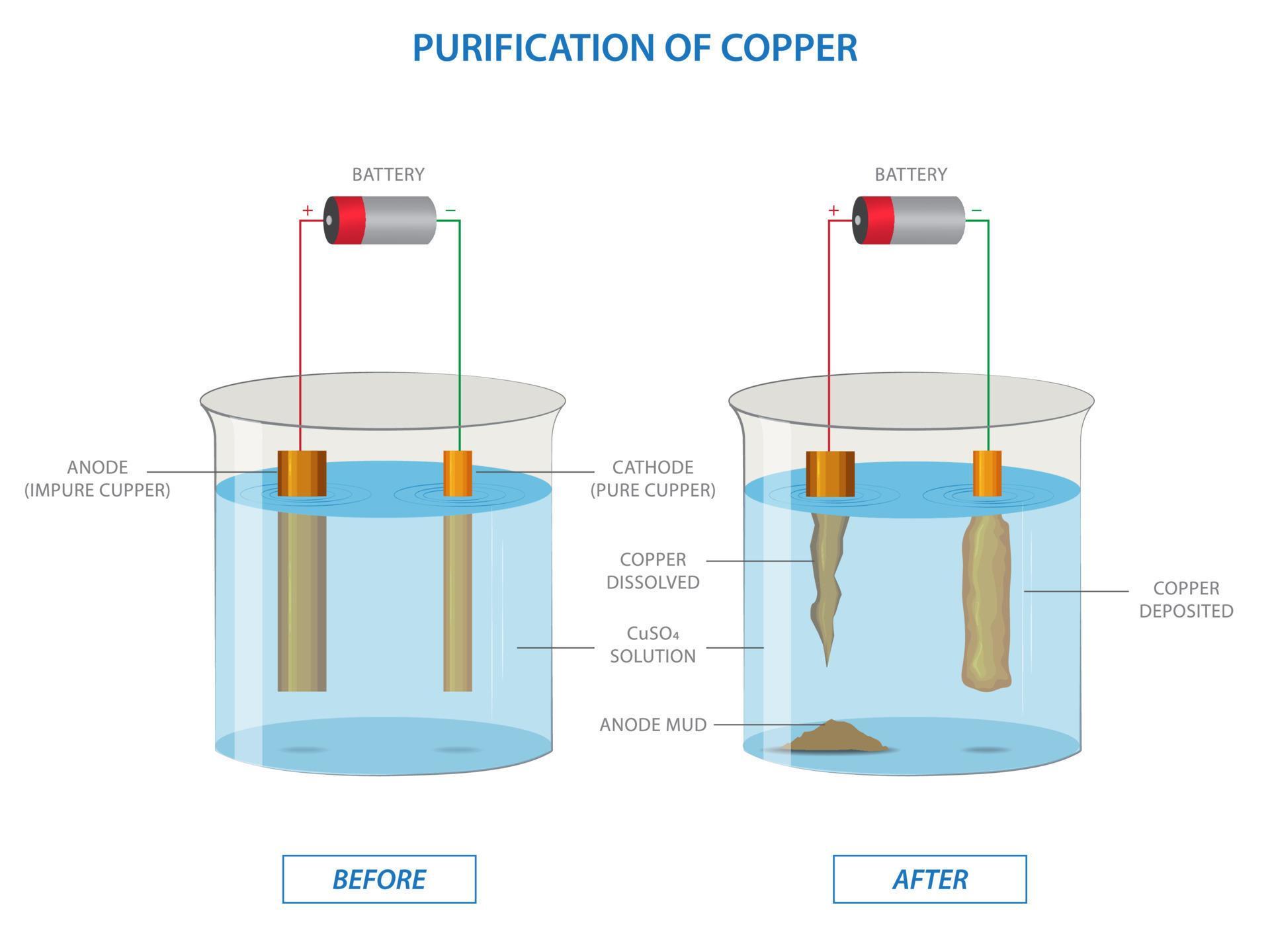
What Is The Solvent Of Copper . The article below highlights some of the important chemical properties of copper. Get periodic table facts on the chemical and physical properties of the element copper. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. Copper is atomic number 29 with element symbol cu. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper is a metal used by people who lived in prehistoric times. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water.
from www.vecteezy.com
Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. The article below highlights some of the important chemical properties of copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper is a metal used by people who lived in prehistoric times. Copper is atomic number 29 with element symbol cu. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on the chemical and physical properties of the element copper.
Electrolysis of copper sulfate solution with impure copper anode and
What Is The Solvent Of Copper For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on the chemical and physical properties of the element copper. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. Copper is a metal used by people who lived in prehistoric times. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper is atomic number 29 with element symbol cu. The article below highlights some of the important chemical properties of copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water.
From www.youtube.com
Electrolysis of Copper Chloride solution National 5 YouTube What Is The Solvent Of Copper It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper is atomic number 29 with element symbol cu. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i). What Is The Solvent Of Copper.
From brainly.in
[Solved] Explain electrolytic refining of copper Brainly.in What Is The Solvent Of Copper Copper is a metal used by people who lived in prehistoric times. Get periodic table facts on the chemical and physical properties of the element copper. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. The article below highlights some of the important chemical properties of copper. It is produced. What Is The Solvent Of Copper.
From sites.google.com
Nomenclature and Predicting Reactions SCH3U CHEMISTRY What Is The Solvent Of Copper Copper is a metal used by people who lived in prehistoric times. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. The article below highlights some of the important chemical properties of copper. Reaction of the ore (over quite a long time and on a huge scale) with a dilute. What Is The Solvent Of Copper.
From www.semanticscholar.org
Figure 1 from Growing role of solvent extraction in copper ores What Is The Solvent Of Copper Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Copper is a. What Is The Solvent Of Copper.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. The article below highlights some of the important chemical properties of copper. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to. What Is The Solvent Of Copper.
From techiescientist.com
Is Copper a Pure Substance? Techiescientist What Is The Solvent Of Copper Copper is atomic number 29 with element symbol cu. The article below highlights some of the important chemical properties of copper. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis. What Is The Solvent Of Copper.
From brainly.com
6. Draw a diagram to illustrate the of copper chloride What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on the chemical and physical properties of the element copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Copper is a metal used by people who lived. What Is The Solvent Of Copper.
From www.chegg.com
Solved The Copper Cycle Objectives To perform series of What Is The Solvent Of Copper It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Copper is atomic number 29 with element symbol cu. For example, if you react copper(i) oxide with. What Is The Solvent Of Copper.
From chemistnotes.com
Copper Detailed explanation, Extraction, Properties, and 6 Uses What Is The Solvent Of Copper The article below highlights some of the important chemical properties of copper. Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper(ii) sulfate is an. What Is The Solvent Of Copper.
From www.greatmining.com
Copper Mining and Extraction Copper Mining Processing What Is The Solvent Of Copper It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. The article below highlights some of the important chemical properties of copper. Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction of mixed copper oxide ores with. What Is The Solvent Of Copper.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate What Is The Solvent Of Copper Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using. What Is The Solvent Of Copper.
From www.btibrands.com
Copper Solvent Battenfeld Technologies What Is The Solvent Of Copper It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper(ii) sulfate is an inorganic compound with the chemical formula cu. What Is The Solvent Of Copper.
From www.coursehero.com
[Solved] Question o When a solution of copper(I) chlorate and a What Is The Solvent Of Copper It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Get periodic table facts on the chemical and physical properties of the element copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. It is produced. What Is The Solvent Of Copper.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. Copper is a metal used by people who lived in prehistoric times. It forms hydrates cuso 4 · n h 2 o , where n can range from 1. What Is The Solvent Of Copper.
From en.wikipedia.org
Copper Wikipedia What Is The Solvent Of Copper The article below highlights some of the important chemical properties of copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper is atomic number 29 with element symbol cu. Copper(ii) sulfate is an inorganic compound with the chemical. What Is The Solvent Of Copper.
From www.presleysoutdoors.com
Copper Solvent IV 80z Presleys Outdoors What Is The Solvent Of Copper The article below highlights some of the important chemical properties of copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on. What Is The Solvent Of Copper.
From blog.thepipingmart.com
5 Physical Properties of Copper What Is The Solvent Of Copper For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. The article below highlights some of the important chemical properties of copper. Reaction of the ore (over quite a long time and on. What Is The Solvent Of Copper.
From scienceinfo.com
Solvent Definition, Types, Incredible Uses, Examples What Is The Solvent Of Copper It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Copper is atomic number 29 with element symbol cu. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on the chemical and physical properties of the element copper. Copper is a metal used. What Is The Solvent Of Copper.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Copper is atomic number 29 with element symbol cu. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Reaction of the ore (over quite a long. What Is The Solvent Of Copper.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog What Is The Solvent Of Copper The article below highlights some of the important chemical properties of copper. Copper is a metal used by people who lived in prehistoric times. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. It forms hydrates cuso 4 ·. What Is The Solvent Of Copper.
From metalprofy.com
Top 5 Best Copper Solvents [January 2024 Review] MetalProfy What Is The Solvent Of Copper For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction. What Is The Solvent Of Copper.
From byjus.com
Electrolysis of cuso4 solution using copper as electrode What Is The Solvent Of Copper Get periodic table facts on the chemical and physical properties of the element copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. The article below highlights some of the important chemical properties of copper. Copper is a metal used by people who lived in. What Is The Solvent Of Copper.
From metalprofy.com
Top 5 Best Copper Solvents [January 2024 Review] MetalProfy What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Copper is a metal used by people who lived in prehistoric times. The article below highlights some of the important chemical properties of copper. It is produced on. What Is The Solvent Of Copper.
From www.creedmoorsports.com
KG12 Copper Solvent 4 Ounce What Is The Solvent Of Copper It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. It forms hydrates cuso 4 ·. What Is The Solvent Of Copper.
From images-of-elements.com
Chemical Elements Copper What Is The Solvent Of Copper The article below highlights some of the important chemical properties of copper. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. It forms hydrates cuso 4 · n h 2 o , where n can range from 1. What Is The Solvent Of Copper.
From www.doubtnut.com
Copper sulphate solution is electrolysed using copper electrodes. What Is The Solvent Of Copper Get periodic table facts on the chemical and physical properties of the element copper. Copper is atomic number 29 with element symbol cu. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution. What Is The Solvent Of Copper.
From www.sciencephoto.com
Copper Sulphate Stock Image C028/1274 Science Photo Library What Is The Solvent Of Copper Get periodic table facts on the chemical and physical properties of the element copper. Copper is atomic number 29 with element symbol cu. The article below highlights some of the important chemical properties of copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Reaction. What Is The Solvent Of Copper.
From metalprofy.com
Top 5 Best Copper Solvents [January 2024 Review] MetalProfy What Is The Solvent Of Copper It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. The article below highlights some of the important chemical properties of copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a. What Is The Solvent Of Copper.
From fineartamerica.com
Copper Ion Solutions Photograph by Andrew Lambert Photography Fine What Is The Solvent Of Copper Copper is a metal used by people who lived in prehistoric times. The article below highlights some of the important chemical properties of copper. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get. What Is The Solvent Of Copper.
From chemistrylabs-2.blogspot.com
Electrolysis Of Copper Sulfate Solution Chemistry Labs What Is The Solvent Of Copper Copper is atomic number 29 with element symbol cu. Get periodic table facts on the chemical and physical properties of the element copper. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7.. What Is The Solvent Of Copper.
From www.bartleby.com
Answered 17 The density of copper is 8.92 g/mL.… bartleby What Is The Solvent Of Copper Copper(ii) sulfate is an inorganic compound with the chemical formula cu so 4. Copper is atomic number 29 with element symbol cu. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. The article below highlights some of the important chemical properties of copper. It forms. What Is The Solvent Of Copper.
From www.proshotproducts.com
Copper Solvent 8 oz. What Is The Solvent Of Copper Copper is atomic number 29 with element symbol cu. It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Get periodic table facts on the chemical and physical properties of the element copper. It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or. What Is The Solvent Of Copper.
From blog.thepipingmart.com
Properties of Copper Wire What Is The Solvent Of Copper It forms hydrates cuso 4 · n h 2 o , where n can range from 1 to 7. Get periodic table facts on the chemical and physical properties of the element copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Copper is a. What Is The Solvent Of Copper.
From www.sportsmansoutdoorsuperstore.com
Pro Shot Copper Solvent 8 oz. Sportsman's Outdoor Superstore What Is The Solvent Of Copper It is produced on a large scale by reduction of mixed copper oxide ores with copper metal or by electrolysis of an aqueous solution of sodium chloride using copper electrodes. Reaction of the ore (over quite a long time and on a huge scale) with a dilute acid. Copper(ii) sulfate is an inorganic compound with the chemical formula cu so. What Is The Solvent Of Copper.
From www.fisherfirearms.com.au
ProShot Copper Solvent IV Fisher Firearms What Is The Solvent Of Copper Copper is a metal used by people who lived in prehistoric times. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Get periodic table facts on the chemical and physical properties of the element copper. Copper(ii) sulfate is an inorganic compound with the chemical formula. What Is The Solvent Of Copper.