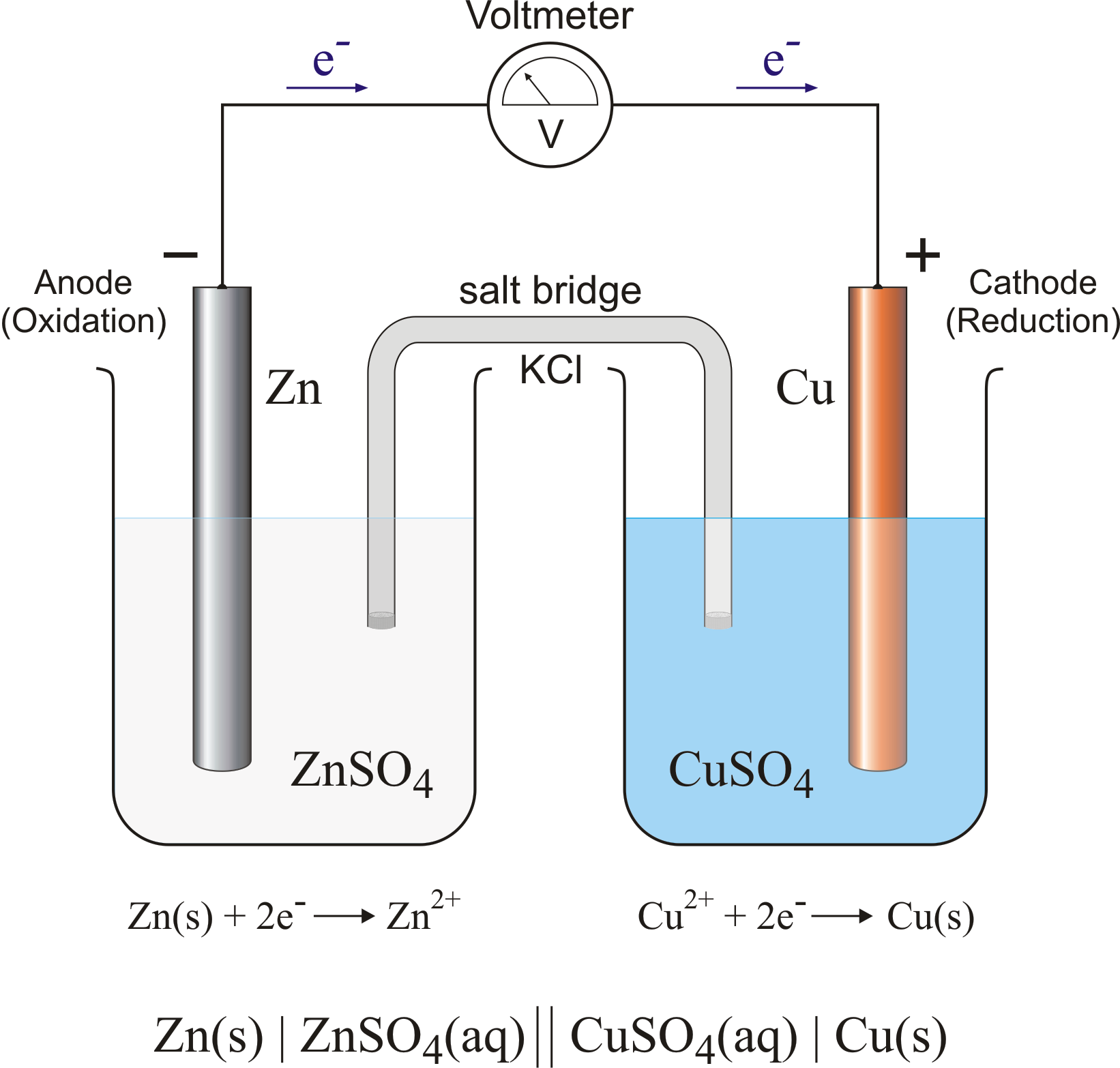
Zinc And Magnesium Voltaic Cell . You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum.
from diagramtabormanages.z21.web.core.windows.net
You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You are given that the electrodes of the galvanic cell are magnesium and zinc.
Diagram Of Voltaic Cell
Zinc And Magnesium Voltaic Cell This arrangement is called a. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You have also identified that the magnesium is the anode and. You are given that the electrodes of the galvanic cell are magnesium and zinc.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells,. Zinc And Magnesium Voltaic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. This arrangement is called a. You have also identified that the magnesium is the anode and. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in. Zinc And Magnesium Voltaic Cell.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all. Zinc And Magnesium Voltaic Cell.
From mavink.com
Zinc Copper Galvanic Cell Zinc And Magnesium Voltaic Cell This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 +. Zinc And Magnesium Voltaic Cell.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while. Zinc And Magnesium Voltaic Cell.
From diagramtabormanages.z21.web.core.windows.net
Diagram Of Voltaic Cell Zinc And Magnesium Voltaic Cell You are given that the electrodes of the galvanic cell are magnesium and zinc. This arrangement is called a. You have also identified that the magnesium is the anode and. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. •in. Zinc And Magnesium Voltaic Cell.
From www.numerade.com
SOLVEDUsing the following data from the Reduction Potentials clearly Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc. Zinc And Magnesium Voltaic Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. This arrangement is called a. •in. Zinc And Magnesium Voltaic Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc And Magnesium Voltaic Cell You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. •in our experiment, the zinc, tin,. Zinc And Magnesium Voltaic Cell.
From stock.adobe.com
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h +. Zinc And Magnesium Voltaic Cell.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. This arrangement is called a. •in. Zinc And Magnesium Voltaic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You are given that the electrodes of the galvanic cell are magnesium and zinc. This arrangement is called a. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 +. Zinc And Magnesium Voltaic Cell.
From wps.prenhall.com
Media Portfolio Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You have also identified that the magnesium is the anode and. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc. Zinc And Magnesium Voltaic Cell.
From electricala2z.com
Voltaic Cell Construction Working Examples Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. This arrangement is called a. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our. Zinc And Magnesium Voltaic Cell.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zinc And Magnesium Voltaic Cell This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc. Zinc And Magnesium Voltaic Cell.
From wisc.pb.unizin.org
D39.4 Introduction to Voltaic Cells Chemistry 109 Fall 2021 Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You have also identified that the magnesium is the anode and. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in. Zinc And Magnesium Voltaic Cell.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Zinc And Magnesium Voltaic Cell This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc. Zinc And Magnesium Voltaic Cell.
From courses.lumenlearning.com
17.2 Galvanic Cells (Voltaic Cells) General College Chemistry II Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h +. Zinc And Magnesium Voltaic Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all. Zinc And Magnesium Voltaic Cell.
From www.bartleby.com
Magnesium metal is oxidized, and silver ions are reduced in a voltaic Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. You have also identified that the magnesium is the anode and. Because the zinc. Zinc And Magnesium Voltaic Cell.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You have also identified that the magnesium is the anode and. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc. Zinc And Magnesium Voltaic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Zinc And Magnesium Voltaic Cell This arrangement is called a. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose. Zinc And Magnesium Voltaic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. This arrangement is called a. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our. Zinc And Magnesium Voltaic Cell.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Zinc And Magnesium Voltaic Cell This arrangement is called a. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while. Zinc And Magnesium Voltaic Cell.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Zinc And Magnesium Voltaic Cell You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the. Zinc And Magnesium Voltaic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. You are given that the electrodes of the galvanic cell are magnesium and zinc. This arrangement is called a. Because the zinc. Zinc And Magnesium Voltaic Cell.
From wisc.pb.unizin.org
Day 39 Voltaic Cells, HalfCell Potentials Chemistry 109 Fall 2021 Zinc And Magnesium Voltaic Cell You are given that the electrodes of the galvanic cell are magnesium and zinc. This arrangement is called a. You have also identified that the magnesium is the anode and. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc. Zinc And Magnesium Voltaic Cell.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while. Zinc And Magnesium Voltaic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose. Zinc And Magnesium Voltaic Cell.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 +. Zinc And Magnesium Voltaic Cell.
From saylordotorg.github.io
Electrochemistry Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while. Zinc And Magnesium Voltaic Cell.
From www.pinterest.com
Kognity Chemistry classroom, Electrochemistry, Chemistry lessons Zinc And Magnesium Voltaic Cell •in our experiment, the zinc, tin, and magnesium all acted as the anode in our voltaic cells, so they all have a greater tendency to lose electrons. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You have also identified. Zinc And Magnesium Voltaic Cell.
From circuitdblandlady.z21.web.core.windows.net
Voltaic Cell Diagram Zinc And Magnesium Voltaic Cell This arrangement is called a. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in our experiment, the zinc, tin, and magnesium all acted as the. Zinc And Magnesium Voltaic Cell.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Zinc And Magnesium Voltaic Cell You have also identified that the magnesium is the anode and. Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. This arrangement is called a. You are given that the electrodes of the galvanic cell are magnesium and zinc. •in. Zinc And Magnesium Voltaic Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Zinc And Magnesium Voltaic Cell Because the zinc electrode in this cell dissolves spontaneously to form zn 2 + (aq) ions while h + (aq) ions are reduced to h 2 at the platinum. You have also identified that the magnesium is the anode and. This arrangement is called a. •in our experiment, the zinc, tin, and magnesium all acted as the anode in our. Zinc And Magnesium Voltaic Cell.