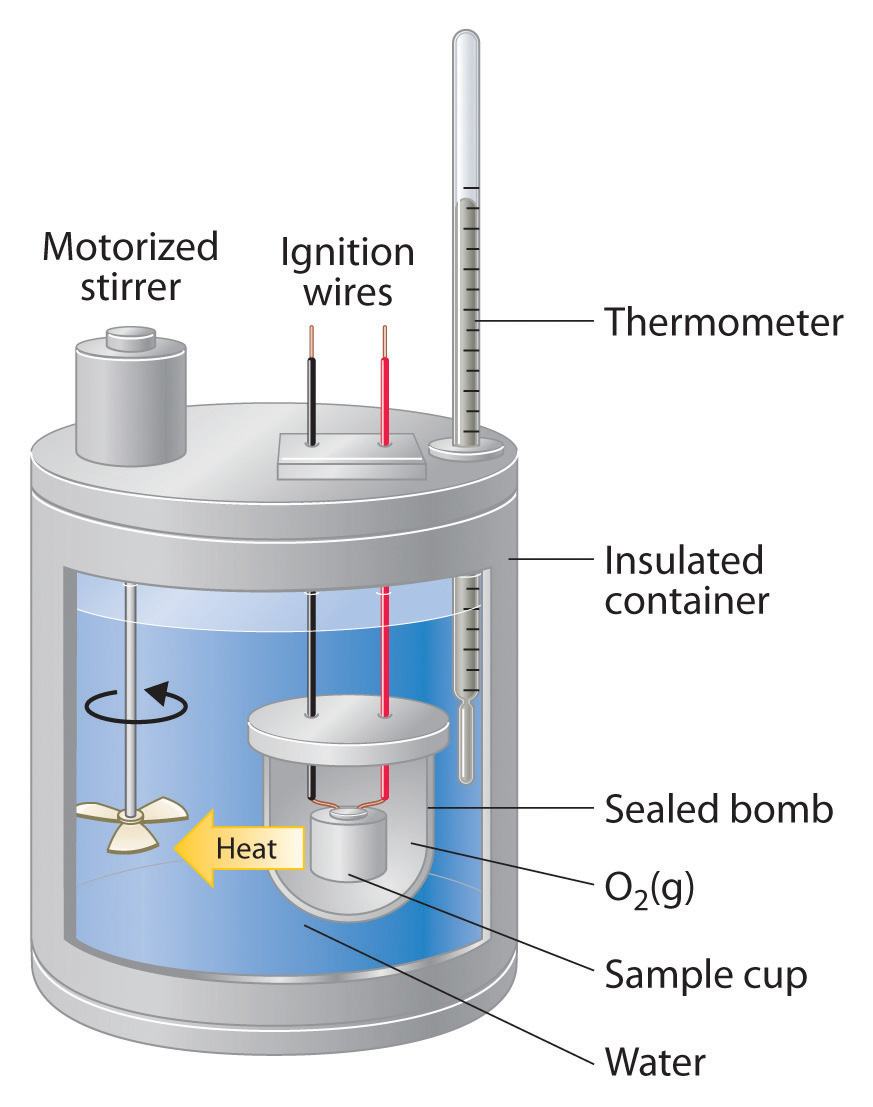
Calorimeter In Chemistry . Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, we will. Chemists use calorimetry to determine the. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. For example, when an exothermic.
from saylordotorg.github.io
Chemists use calorimetry to determine the. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. In this article, we will. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different.
Calorimetry
Calorimeter In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Chemists use calorimetry to determine the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The process of measuring this heat is called calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. In this article, we will.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. In this article, we will. Calorimetry measures enthalpy changes during chemical processes, where the. Calorimeter In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In this article, we will. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The process of measuring this heat is called calorimetry. Chemists use calorimetry to determine the. A calorimeter is a device used. Calorimeter In Chemistry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimeter In Chemistry For example, when an exothermic. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. In this article, we will. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a. Calorimeter In Chemistry.
From opentextbc.ca
5.2 Calorimetry Chemistry Calorimeter In Chemistry Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this. Calorimeter In Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter In Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is the measurement of the transfer of heat into or. Calorimeter In Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter In Chemistry A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. Calorimeter In Chemistry.
From users.highland.edu
Calorimetry Calorimeter In Chemistry For example, when an exothermic. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Chemists use calorimetry to determine the. In this article, we will. The process of measuring this heat is called calorimetry. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released. Calorimeter In Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter In Chemistry For example, when an exothermic. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. The process of measuring this heat is called calorimetry. Calorimetry is a field of thermochemistry. Calorimeter In Chemistry.
From www.animalia-life.club
Calorimeter Diagram Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to. Calorimeter In Chemistry.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimeter In Chemistry In this article, we will. For example, when an exothermic. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the transfer of heat into or out of a. Calorimeter In Chemistry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of. Calorimeter In Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. In this article, we will. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. Calorimeter In Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The process of measuring this heat is called calorimetry. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter In Chemistry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimeter In Chemistry For example, when an exothermic. In this article, we will. Chemists use calorimetry to determine the. The process of measuring this heat is called calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during. Calorimeter In Chemistry.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, we will. Calorimetry is a field of thermochemistry that measures the amount of heat involved in. Calorimeter In Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Calorimeter In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. In this article, we will. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We will apply calorimetry to two different thermodynamic. Calorimeter In Chemistry.
From byjus.com
The calorimeter is commonly made up of……..because it has……. Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical. Calorimeter In Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter In Chemistry Chemists use calorimetry to determine the. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a. Calorimeter In Chemistry.
From www.terraanaliz.com
Atlas HD Reaction Calorimeter Batch Chemistry Calorimeter In Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. The process of measuring this. Calorimeter In Chemistry.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter In Chemistry In this article, we will. Chemists use calorimetry to determine the. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. The process of measuring this. Calorimeter In Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter In Chemistry Chemists use calorimetry to determine the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical. Calorimeter In Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter In Chemistry Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example,. Calorimeter In Chemistry.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter In Chemistry We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. The process of measuring this heat is called calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimeter In Chemistry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter In Chemistry Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. The process of measuring this heat is called calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry measures enthalpy changes during chemical. Calorimeter In Chemistry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to. Calorimeter In Chemistry.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimeter In Chemistry The process of measuring this heat is called calorimetry. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is a field of thermochemistry that measures the amount of heat involved. Calorimeter In Chemistry.
From ar.inspiredpencil.com
Calorimeter Diagram Calorimeter In Chemistry We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. The process of measuring this heat is called calorimetry. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the heat flow. Calorimeter In Chemistry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimeter In Chemistry In this article, we will. For example, when an exothermic. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter In Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Calorimeters is. Calorimeter In Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Calorimeter In Chemistry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Chemists use calorimetry to determine the. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released. Calorimeter In Chemistry.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. We will apply calorimetry to two different thermodynamic processes, that of transference. Calorimeter In Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimeter In Chemistry Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Chemists use calorimetry to determine the.. Calorimeter In Chemistry.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Calorimeter In Chemistry A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different. For example, when an exothermic. In this article, we will. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimeter In Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter In Chemistry For example, when an exothermic. In this article, we will. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Chemists use calorimetry to determine the. A calorimeter is. Calorimeter In Chemistry.
From saylordotorg.github.io
Calorimetry Calorimeter In Chemistry Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter In Chemistry.