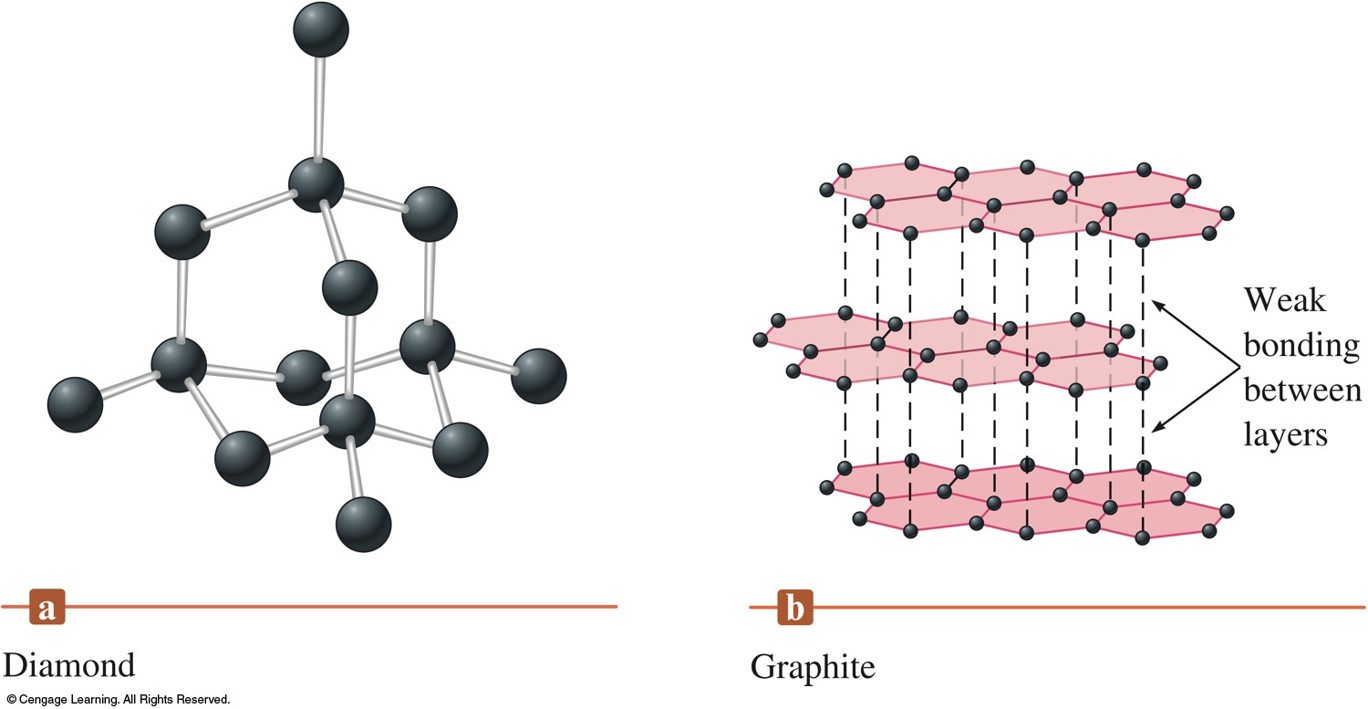
Graphite Carbon Structure . Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite is one of the most frequent allotropes of carbon. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. This crystal carbon has a structure that is planar and layered. Graphene is the term used to denote each layer. These rings are attached to one another on their edges. Graphite, mineral consisting of carbon. Layers of fused rings can be modeled as. Carbon has an electronic arrangement of 2,4.
from shaunmwilliams.com
Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Layers of fused rings can be modeled as. Graphite, mineral consisting of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is one of the most frequent allotropes of carbon. These rings are attached to one another on their edges. Graphene is the term used to denote each layer. This crystal carbon has a structure that is planar and layered. Carbon has an electronic arrangement of 2,4. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining.
Chapter 10 Presentation
Graphite Carbon Structure Layers of fused rings can be modeled as. Graphite, mineral consisting of carbon. Graphite is one of the most frequent allotropes of carbon. These rings are attached to one another on their edges. Carbon has an electronic arrangement of 2,4. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Graphene is the term used to denote each layer. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Layers of fused rings can be modeled as. This crystal carbon has a structure that is planar and layered.
From www.alamy.com
modification of carbon molecule structure of diamond, graphite and Graphite Carbon Structure It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Carbon has an electronic arrangement of 2,4. This crystal carbon has a structure that is planar and layered. These rings are attached to one another on their edges. Graphite is one of the most frequent allotropes of carbon. Layers. Graphite Carbon Structure.
From byjus.com
Describe the structure of Graphite. Graphite Carbon Structure Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite, mineral consisting of carbon. This crystal carbon has a structure that is planar and layered. These rings are attached to one another on their edges. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry. Graphite Carbon Structure.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080 Graphite Carbon Structure Layers of fused rings can be modeled as. Graphite is one of the most frequent allotropes of carbon. This crystal carbon has a structure that is planar and layered. Carbon has an electronic arrangement of 2,4. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Each carbon atom. Graphite Carbon Structure.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID2754504 Graphite Carbon Structure Graphite is one of the most frequent allotropes of carbon. Carbon has an electronic arrangement of 2,4. Layers of fused rings can be modeled as. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. It is also the most stable allotrope of. Graphite Carbon Structure.
From www.youtube.com
Structure of Graphite YouTube Graphite Carbon Structure Graphene is the term used to denote each layer. Graphite, mineral consisting of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Layers of fused rings can be modeled as. These rings are attached to one another on their edges. Graphite. Graphite Carbon Structure.
From www.acsmaterial.com
Graphene Super Strength for the 21st Century Graphite Carbon Structure This crystal carbon has a structure that is planar and layered. These rings are attached to one another on their edges. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is one of the most frequent allotropes of carbon. Layers of. Graphite Carbon Structure.
From www.savemyexams.com
Structure & Physical Properties OCR A Level Chemistry Revision Notes 2017 Graphite Carbon Structure This crystal carbon has a structure that is planar and layered. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite. Graphite Carbon Structure.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.5.7 Covalent Structures翰林国际教育 Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. Graphene is the term used to denote each layer. Graphite, mineral consisting of carbon. Layers of fused rings can be modeled as. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is one of. Graphite Carbon Structure.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Graphite Carbon Structure Layers of fused rings can be modeled as. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. This crystal carbon has a structure that is planar and layered. Graphite, mineral consisting of carbon. Graphite is one of the most frequent allotropes of carbon. Graphene is the term used to denote. Graphite Carbon Structure.
From www.britannica.com
Carbon Structure of carbon allotropes Graphite Carbon Structure Graphite is one of the most frequent allotropes of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphene is the term used to denote each layer. This crystal carbon has a structure that is planar and layered. Graphite, mineral consisting of carbon. It is also the most stable. Graphite Carbon Structure.
From worldgeoinfo.blogspot.com
Definition And Classification of Rocks, Mountain and Plateaus Basic Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. Layers of fused rings can be modeled as. Graphite is one of the most frequent allotropes of carbon. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. This crystal carbon has a structure that is planar and layered. Graphite, mineral consisting. Graphite Carbon Structure.
From ar.inspiredpencil.com
Carbon Structure Of Diamond And Graphite Graphite Carbon Structure Graphite, mineral consisting of carbon. This crystal carbon has a structure that is planar and layered. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the. Graphite Carbon Structure.
From brainly.in
draw the structure of Graphite Brainly.in Graphite Carbon Structure These rings are attached to one another on their edges. Graphene is the term used to denote each layer. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one. Graphite Carbon Structure.
From www.nisenet.org
Scientific Image Graphite models NISE Network Graphite Carbon Structure These rings are attached to one another on their edges. Graphite, mineral consisting of carbon. Layers of fused rings can be modeled as. Graphene is the term used to denote each layer. Graphite is one of the most frequent allotropes of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers. Graphite Carbon Structure.
From substech.com
Graphite [SubsTech] Graphite Carbon Structure Layers of fused rings can be modeled as. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. This crystal carbon has a structure that is planar and layered. Graphene. Graphite Carbon Structure.
From ar.inspiredpencil.com
Diamond And Graphite Structure Graphite Carbon Structure Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is one of the most frequent allotropes of carbon. These rings are attached to one another on their edges. Carbon has an electronic arrangement of 2,4. It is also the most stable. Graphite Carbon Structure.
From sajhanotes.com
Carbon NEB Grade 11 Notes Chemistry Sajha Notes Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. Layers of fused rings can be modeled as. This crystal carbon has a structure that is planar and layered. These rings are attached to one another on their edges. Graphite is one of the most frequent allotropes of carbon. Each carbon atom in graphite is able to form three covalent bonds to other. Graphite Carbon Structure.
From shaunmwilliams.com
Chapter 10 Presentation Graphite Carbon Structure Graphene is the term used to denote each layer. Graphite is one of the most frequent allotropes of carbon. Carbon has an electronic arrangement of 2,4. Graphite, mineral consisting of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. These rings. Graphite Carbon Structure.
From www.animalia-life.club
Graphite Crystal Structure Graphite Carbon Structure Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. This crystal carbon has a structure that is planar and layered. Layers of fused rings can be modeled as. It is also the most stable allotrope of carbon and is thus utilized in. Graphite Carbon Structure.
From www.animalia-life.club
Structure Of Diamond Graphite Carbon Structure Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite, mineral consisting of carbon. Layers of fused rings can be modeled as. Graphite is one of the most frequent allotropes of carbon. These rings are attached to one another on their edges.. Graphite Carbon Structure.
From stock.adobe.com
Vecteur Stock Graphite layers, threedimensional, schematic diagram Graphite Carbon Structure Graphite, mineral consisting of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is one of the most frequent allotropes of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein,. Graphite Carbon Structure.
From www.thesciencehive.co.uk
Structure and Bonding of Carbon (AQA) — the science sauce Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. These rings are attached to one another on their edges. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite, mineral consisting of carbon. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of. Graphite Carbon Structure.
From www.shutterstock.com
Graphite Structure Allotrope Carbon Vector Illustration Stock Vector Graphite Carbon Structure Graphite is one of the most frequent allotropes of carbon. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Carbon has an electronic arrangement of 2,4. This crystal carbon has a structure that is planar and layered. Graphite, mineral consisting of carbon. Each carbon atom in graphite is. Graphite Carbon Structure.
From www.researchgate.net
Graphene is a 2D building material for carbon materials of all other Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. Graphite, mineral consisting of carbon. Layers of fused rings can be modeled as. Graphene is the term used to denote each layer. This crystal carbon has a structure that is planar and layered. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons,. Graphite Carbon Structure.
From glossary.periodni.com
Graphite Chemistry Dictionary & Glossary Graphite Carbon Structure Layers of fused rings can be modeled as. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Graphene is the term used to denote each layer. Graphite is one of the most frequent allotropes of carbon. Graphite has a greasy feel and leaves a black mark, thus the. Graphite Carbon Structure.
From ar.inspiredpencil.com
Carbon Atom Structure Model Graphite Carbon Structure Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Layers of fused rings can be modeled as. Graphite is one of the most frequent allotropes of carbon. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Carbon has. Graphite Carbon Structure.
From www.dreamstime.com
107 Graphite Layer Photos Free & RoyaltyFree Stock Photos from Graphite Carbon Structure Graphite, mineral consisting of carbon. Carbon has an electronic arrangement of 2,4. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. These rings are attached to one another on their edges. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb. Graphite Carbon Structure.
From parcoscientific.com
Graphite Molecular Model Molecular Models Chemistry Graphite Carbon Structure Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Carbon has an electronic arrangement of 2,4. Layers of fused rings can be modeled as. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein,. Graphite Carbon Structure.
From socratic.org
What are diamond and graphite in relation to carbon? Socratic Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. This crystal carbon has a structure that is planar and layered. Layers of fused rings can be modeled as. Each carbon atom in graphite is able to form three. Graphite Carbon Structure.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Graphite Carbon Structure These rings are attached to one another on their edges. Graphite is one of the most frequent allotropes of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Layers of fused rings can be modeled as. Graphene is the term used to denote each layer. It is also the. Graphite Carbon Structure.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Graphite Carbon Structure Graphene is the term used to denote each layer. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. This crystal carbon has a structure that is planar and layered. Graphite is one of the most frequent allotropes of carbon. These rings are attached to one. Graphite Carbon Structure.
From www.animalia-life.club
Graphite Atom Structure Graphite Carbon Structure This crystal carbon has a structure that is planar and layered. Graphene is the term used to denote each layer. Carbon has an electronic arrangement of 2,4. Graphite is one of the most frequent allotropes of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite, mineral consisting of. Graphite Carbon Structure.
From www.slideserve.com
PPT Why do atoms form bonds? PowerPoint Presentation, free download Graphite Carbon Structure Carbon has an electronic arrangement of 2,4. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a greasy feel. Graphite Carbon Structure.
From mwi-inc.com
structure of graphite MWI, Inc. Graphite Carbon Structure These rings are attached to one another on their edges. This crystal carbon has a structure that is planar and layered. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Carbon has an electronic arrangement of 2,4. Graphene is the term used to denote each layer. Graphite has. Graphite Carbon Structure.
From lpmmc.grenoble.cnrs.fr
Laboratoire de Physique et Modélisation des Milieux Condensés Graphite Carbon Structure Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. These rings are attached to one another on their edges. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining. Graphite is. Graphite Carbon Structure.