What Is The Boiling Point Of Water Inside The Pressure Cooker . When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). Raises the boiling point of the water in the pot. When the vessel is heated, water vapor increases the pressure inside the vessel; As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; But with the steam’s pressure now the boiling point can get as high as 250°f. What happens to the boiling point in a pressure cooker? At standard atmospheric pressure, the boiling point of water is 212 f (100 c). This higher temperature cooks food faster than conventional boiling or steaming. But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or This is why food gets Normally, water boils at 100 c (212 f) at sea level. The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container.
from fyzikalnipokusy.cz
The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; This is why food gets But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or This higher temperature cooks food faster than conventional boiling or steaming. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). Normally, water boils at 100 c (212 f) at sea level. What happens to the boiling point in a pressure cooker? When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f).
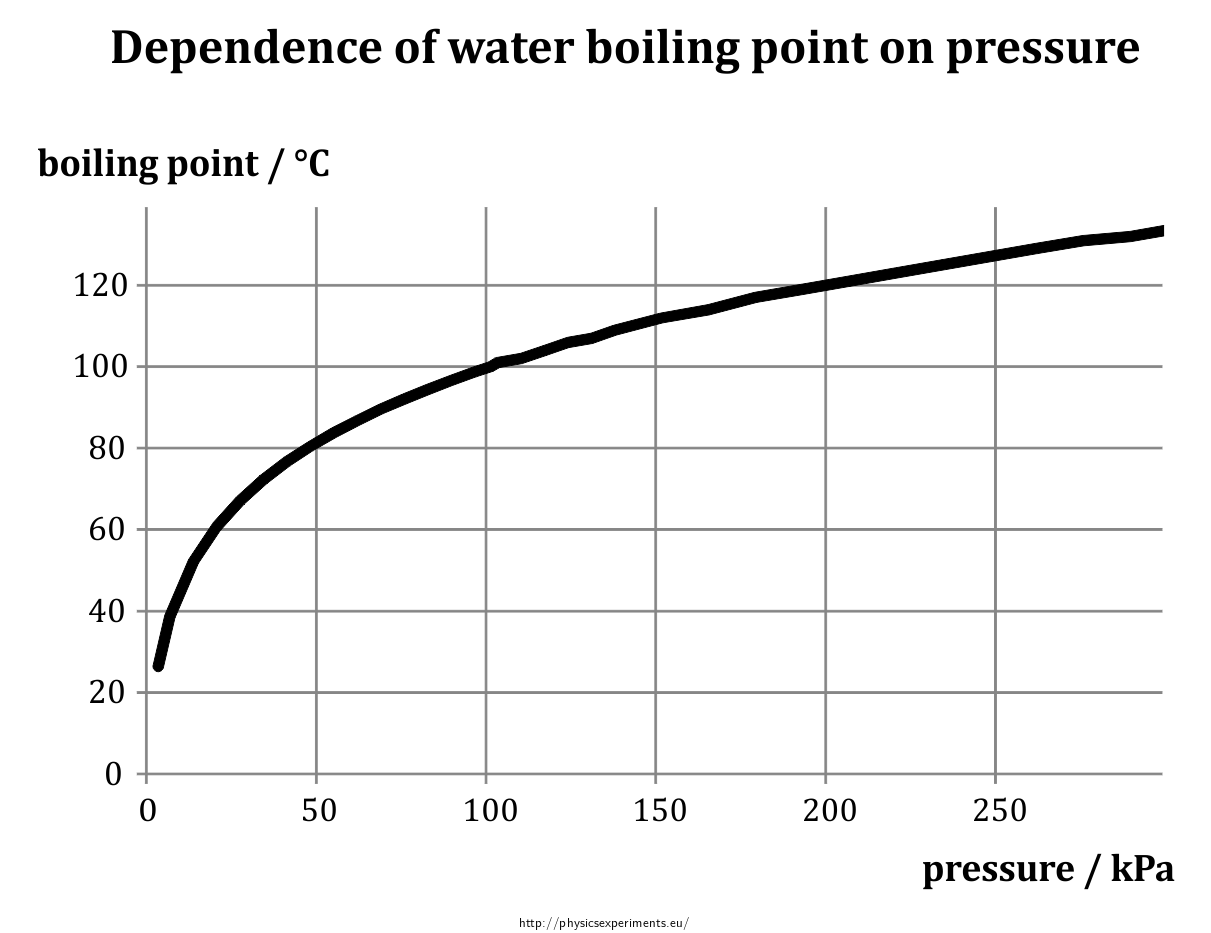
Dependence of Boiling Point of Water on Pressure — Collection of
What Is The Boiling Point Of Water Inside The Pressure Cooker The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. This is why food gets When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). Normally, water boils at 100 c (212 f) at sea level. As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; But with the steam’s pressure now the boiling point can get as high as 250°f. This higher temperature cooks food faster than conventional boiling or steaming. The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). This higher heat helps the food to cook faster. What happens to the boiling point in a pressure cooker? Raises the boiling point of the water in the pot. At standard atmospheric pressure, the boiling point of water is 212 f (100 c).
From fyzikalnipokusy.cz
Dependence of Boiling Point of Water on Pressure — Collection of What Is The Boiling Point Of Water Inside The Pressure Cooker The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; Normally, water boils at 100 c (212 f) at sea level. But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Boiling Point Of Water Inside The Pressure Cooker Normally, water boils at 100 c (212 f) at sea level. This higher temperature cooks food faster than conventional boiling or steaming. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c /. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Boiling Point Of Water Inside The Pressure Cooker This is why food gets What happens to the boiling point in a pressure cooker? The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? What Is The Boiling Point Of Water Inside The Pressure Cooker This higher heat helps the food to cook faster. Raises the boiling point of the water in the pot. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. However, inside a pressure cooker, the. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From ar.inspiredpencil.com
Boiling Point Of Water For Kids What Is The Boiling Point Of Water Inside The Pressure Cooker But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or Normally, water boils at 100 c (212 f) at sea level. What happens to the boiling point in a pressure cooker? When the pressure increases, the temperature of the water and steam increases to the normal. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.animalia-life.club
Boiling Point Of Water Examples What Is The Boiling Point Of Water Inside The Pressure Cooker This higher temperature cooks food faster than conventional boiling or steaming. However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. When cooking something wet, like a stew or steamed vegetables, the heat. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is The Boiling Point Of Water Inside The Pressure Cooker Raises the boiling point of the water in the pot. But with the steam’s pressure now the boiling point can get as high as 250°f. This higher heat helps the food to cook faster. This higher temperature cooks food faster than conventional boiling or steaming. When the vessel is heated, water vapor increases the pressure inside the vessel; The ideal. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is The Boiling Point Of Water Inside The Pressure Cooker When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). At standard atmospheric pressure, the boiling point of water is 212 f (100 c). Raises the boiling point of the water in the pot. Normally, water boils at 100 c (212 f) at sea level. This. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From ar.inspiredpencil.com
Boiling Point Of Water For Kids What Is The Boiling Point Of Water Inside The Pressure Cooker But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or This higher heat helps the food to cook faster. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). When cooking something wet, like a stew or steamed vegetables, the heat of. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From mavink.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water Inside The Pressure Cooker But with the steam’s pressure now the boiling point can get as high as 250°f. What happens to the boiling point in a pressure cooker? This is why food gets When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. Normally, water boils at 100 c (212 f) at. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Is The Boiling Point Of Water Inside The Pressure Cooker The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). But with the. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Is The Boiling Point Of Water Inside The Pressure Cooker This higher temperature cooks food faster than conventional boiling or steaming. As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. This is why food gets This higher heat helps the food to cook faster. When the pressure increases, the temperature of the water and steam increases to the normal. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From stravaganzastravaganza.blogspot.com
S T R A V A G A N Z A HISTORY OF PRESSURE COOKER What Is The Boiling Point Of Water Inside The Pressure Cooker However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. Normally, water boils at 100 c (212 f) at sea level. The ideal gas law operates in a pressure cooker because the steam cannot. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From mungfali.com
Refrigerant Boiling Point Chart What Is The Boiling Point Of Water Inside The Pressure Cooker Normally, water boils at 100 c (212 f) at sea level. When the vessel is heated, water vapor increases the pressure inside the vessel; When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. As the pressure increases, the boiling point of water also increases, reaching up to 130o. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is The Boiling Point Of Water Inside The Pressure Cooker When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. When the vessel is heated, water vapor increases the pressure inside the vessel; Raises the boiling point of the water in the pot. The pressure increase in turn raises the boiling point of water, which normally limits the cooking. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Is The Boiling Point Of Water Inside The Pressure Cooker What happens to the boiling point in a pressure cooker? When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. But with the steam’s pressure now the boiling point can get as high as 250°f. This higher heat helps the food to cook faster. As the pressure increases, the. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis What Is The Boiling Point Of Water Inside The Pressure Cooker What happens to the boiling point in a pressure cooker? As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. This is why food gets But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or But. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From sciencenotes.org
How to Boil Water at Room Temperature What Is The Boiling Point Of Water Inside The Pressure Cooker What happens to the boiling point in a pressure cooker? Normally, water boils at 100 c (212 f) at sea level. However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). This higher heat helps the food to cook faster. The ideal gas law operates in a pressure cooker because the steam cannot escape. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.pinterest.com
Atmospheric pressure and boiling point of water table Boiling point What Is The Boiling Point Of Water Inside The Pressure Cooker But with the steam’s pressure now the boiling point can get as high as 250°f. This higher heat helps the food to cook faster. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From mungfali.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water Inside The Pressure Cooker However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). This higher heat helps the food to cook faster. The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. When the vessel is heated, water vapor increases the pressure inside the vessel;. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Is The Boiling Point Of Water Inside The Pressure Cooker This higher temperature cooks food faster than conventional boiling or steaming. But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or When the vessel is heated, water vapor increases the pressure inside the vessel; The pressure increase in turn raises the boiling point of water, which. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From usermanualcommoner.z21.web.core.windows.net
Boiling Water Tap Diagram What Is The Boiling Point Of Water Inside The Pressure Cooker When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). This higher heat helps the food to cook faster. What happens to the boiling point in a pressure cooker? The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From collinewelshs.blob.core.windows.net
Pressure Cooker Boiling Point Of Water at collinewelshs blog What Is The Boiling Point Of Water Inside The Pressure Cooker But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or This higher temperature cooks food faster than conventional boiling or steaming. When the vessel is heated, water vapor increases the pressure inside the vessel; Raises the boiling point of the water in the pot. As the. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From powellcovelaw1980.blogspot.com
What Is The Change In The Boiling Point Of Water When The Pressure Is What Is The Boiling Point Of Water Inside The Pressure Cooker When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. What happens to the boiling point in a pressure cooker? But with the steam’s pressure. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.numerade.com
SOLVED Based on the figure below, what is the boiling point of water What Is The Boiling Point Of Water Inside The Pressure Cooker When the vessel is heated, water vapor increases the pressure inside the vessel; However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). Normally, water boils at 100 c (212. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs What Is The Boiling Point Of Water Inside The Pressure Cooker Normally, water boils at 100 c (212 f) at sea level. When the vessel is heated, water vapor increases the pressure inside the vessel; When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. This higher temperature cooks food faster than conventional boiling or steaming. As the pressure increases,. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.worksheetsplanet.com
What is Boiling Point What Is The Boiling Point Of Water Inside The Pressure Cooker But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. The ideal gas law operates in a pressure cooker because the steam cannot escape the. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.worldatlas.com
What is the Boiling Point of Water? WorldAtlas What Is The Boiling Point Of Water Inside The Pressure Cooker What happens to the boiling point in a pressure cooker? But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or Normally, water boils at 100 c (212 f) at sea level. When the pressure increases, the temperature of the water and steam increases to the normal. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From mavink.com
Water Boiling Pressure Chart What Is The Boiling Point Of Water Inside The Pressure Cooker Normally, water boils at 100 c (212 f) at sea level. As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. When the vessel is heated, water vapor increases the pressure inside the vessel; Raises the boiling point of the water in the pot. This is why food gets This. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.numerade.com
SOLVED Foods cook faster when placed in a pressure cooker. This is What Is The Boiling Point Of Water Inside The Pressure Cooker As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. Raises the boiling point of the water in the pot. However, inside a pressure cooker, the boiling point can. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.reddit.com
What is the melting point of iron in space; What is the boiling point What Is The Boiling Point Of Water Inside The Pressure Cooker But in a standard american pressure cooker, the pressure reaches 1 atm or 15 psi (pounds per square inch) above standard atmospheric pressure*, or However, inside a pressure cooker, the boiling point can increase to around 120 c (248 f). What happens to the boiling point in a pressure cooker? This is why food gets But with the steam’s pressure. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From eatingrules.com
The Science of Pressure Cookers Eating Rules What Is The Boiling Point Of Water Inside The Pressure Cooker The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. When the vessel is heated, water vapor increases the pressure inside the vessel; This is why food gets This higher temperature cooks food faster than conventional boiling or steaming. Normally, water boils at 100 c (212 f) at. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Is The Boiling Point Of Water Inside The Pressure Cooker As the pressure increases, the boiling point of water also increases, reaching up to 130o celsius, rather than 100o celsius. Raises the boiling point of the water in the pot. The pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 c / 212 f (at sea level; However,. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.gkseries.com
The boiling of water inside the pressure cooker is What Is The Boiling Point Of Water Inside The Pressure Cooker When the vessel is heated, water vapor increases the pressure inside the vessel; The ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. At standard atmospheric pressure, the boiling point of water is 212 f (100 c). However, inside a pressure cooker, the boiling point can increase to. What Is The Boiling Point Of Water Inside The Pressure Cooker.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Boiling Point Of Water Inside The Pressure Cooker When cooking something wet, like a stew or steamed vegetables, the heat of your cooking is limited to the boiling point of water (212°f). This higher heat helps the food to cook faster. When the pressure increases, the temperature of the water and steam increases to the normal boiling point of 212 degrees fahrenheit. The pressure increase in turn raises. What Is The Boiling Point Of Water Inside The Pressure Cooker.