Buffer Example Problems . Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. Use the titrations on the web site (move the slider bar to. Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Systematic solution to buffer problems. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared.
from www.slideserve.com
Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. Systematic solution to buffer problems. Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared.
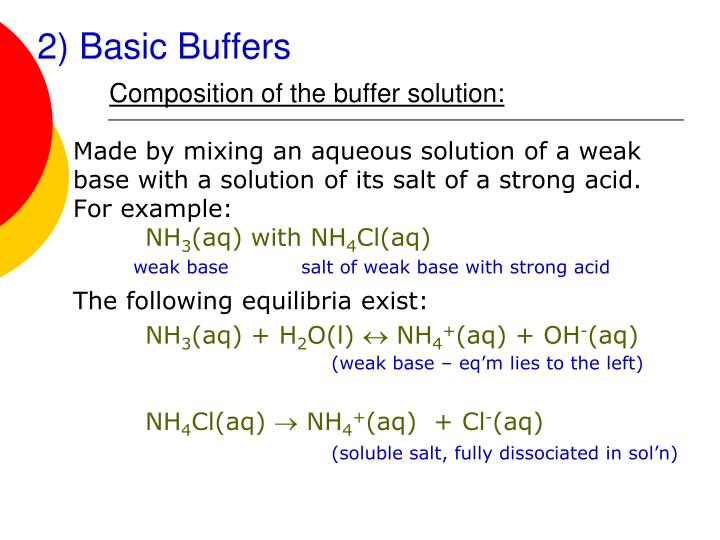
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Systematic solution to buffer problems. Use the titrations on the web site (move the slider bar to.
From www.youtube.com
17.2 Buffer Example Problem YouTube Buffer Example Problems A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Buffer Example Problems.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. A buffer is prepared containing 1.00 m acetic acid and. Buffer Example Problems.
From www.youtube.com
Calculate Concentrations in a Buffer (Example Problem) YouTube Buffer Example Problems Use the titrations on the web site (move the slider bar to. Calculate the ph of an unbuffered 0.010m acetic acid solution. Systematic solution to buffer problems. 1) to solve the above. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. A. Buffer Example Problems.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Buffer Example Problems Systematic solution to buffer problems. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Use the titrations on the web site (move the slider bar to. 1) to solve the above. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate.. Buffer Example Problems.
From www.wizeprep.com
Buffers Problem Type 2 Wize University Chemistry Textbook Wizeprep Buffer Example Problems Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Use the titrations on the web site (move the slider bar to. Systematic solution to buffer problems. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above.. Buffer Example Problems.
From www.artofit.org
Buffer solutions examples and buffer chemistry notes Artofit Buffer Example Problems Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. 1) to solve the above. Calculate the ph of. Buffer Example Problems.
From criticalthinking.cloud
how to solve buffer problems chemistry Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Systematic solution to buffer problems. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). 1) to solve the above. Calculate the ph. Buffer Example Problems.
From www.scienceforums.net
buffer problem Chemistry Science Forums Buffer Example Problems 1) to solve the above. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the. Buffer Example Problems.
From criticalthinking.cloud
how to solve buffer problems chemistry Buffer Example Problems Use the titrations on the web site (move the slider bar to. Calculate the ph of an unbuffered 0.010m acetic acid solution. 1) to solve the above. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a. Buffer Example Problems.
From criticalthinking.cloud
how to solve buffer problems chemistry Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate the ph of an unbuffered 0.010m acetic acid solution.. Buffer Example Problems.
From www.youtube.com
Buffer Problem pOH of a buffer solution with the HendersonHasselbach Buffer Example Problems Systematic solution to buffer problems. 1) to solve the above. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Calculate the ph of an unbuffered 0.010m acetic acid solution. Use the titrations on the web site (move the slider bar to. Buffer. Buffer Example Problems.
From www.youtube.com
pH of Buffer Solution (Example) YouTube Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Systematic solution to buffer problems. 1) to solve the above. Use the titrations. Buffer Example Problems.
From www.youtube.com
17.2 Buffer Example Problem YouTube Buffer Example Problems A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Use the titrations on the web site (move the slider bar to. This is a summary practice. Buffer Example Problems.
From www.youtube.com
Biochemistry pH and Buffer Problems YouTube Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. 1) to solve the above. Calculate the ph of an. Buffer Example Problems.
From criticalthinking.cloud
how to solve buffer problems chemistry Buffer Example Problems Systematic solution to buffer problems. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. 1) to solve the above. Use the titrations. Buffer Example Problems.
From www.youtube.com
Chem 1BLec 33Buffers Part 5 (Practice Problems) YouTube Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Systematic solution to buffer problems. Calculate the ph of an unbuffered 0.010m acetic acid solution. Use the titrations on the web site (move the slider bar to. A buffer is prepared containing 1.00. Buffer Example Problems.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Example Problems Use the titrations on the web site (move the slider bar to. Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. A buffer is prepared containing 1.00 m acetic acid and 1.00. Buffer Example Problems.
From www.youtube.com
The Bounded Buffer Problem YouTube Buffer Example Problems 1) to solve the above. Calculate the ph of an unbuffered 0.010m acetic acid solution. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Buffer solutions resist a change in ph when. Buffer Example Problems.
From www.youtube.com
Practice Buffer Action problems YouTube Buffer Example Problems Use the titrations on the web site (move the slider bar to. Calculate the ph of an unbuffered 0.010m acetic acid solution. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)).. Buffer Example Problems.
From www.youtube.com
basic buffer problem YouTube Buffer Example Problems Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Buffer solutions resist a change in ph when small amounts of. Buffer Example Problems.
From studylib.net
A4 — BUFFER PROBLEMS (EXAMPLES WITH SOLUTIONS Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. Buffer Example Problems.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Example Problems Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l. Buffer Example Problems.
From www.studocu.com
Buffer Example Problem BUFFER EXAMPLE PROBLEM Prepare 100ml of a 0 Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate the ph of an unbuffered 0.010m acetic acid solution. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Use the titrations. Buffer Example Problems.
From www.studocu.com
Bounded Buffer Problem Bounded Buffer Problem Bounded buffer problem Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Systematic solution to buffer problems. 1) to solve the above. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared.. Buffer Example Problems.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Example Problems Calculate the ph of an unbuffered 0.010m acetic acid solution. Use the titrations on the web site (move the slider bar to. 1) to solve the above. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a. Buffer Example Problems.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Example Problems 1) to solve the above. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Systematic solution to buffer problems. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Buffer solutions resist a change in ph when small. Buffer Example Problems.
From www.slideserve.com
PPT Chapter 2 Processes & Threads PowerPoint Presentation, free Buffer Example Problems Use the titrations on the web site (move the slider bar to. Systematic solution to buffer problems. Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. A buffer is prepared containing 1.00. Buffer Example Problems.
From www.youtube.com
AP Chemistry review of buffer question YouTube Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate the ph of an unbuffered 0.010m acetic acid solution. Use the titrations on the web site (move the slider bar to. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Systematic. Buffer Example Problems.
From www.youtube.com
Buffer Solution Questions Part2 IIT JEE NEET YouTube Buffer Example Problems A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. This is a summary practice problem set on buffer solutions aimed to help identify. Buffer Example Problems.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Buffer Example Problems Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). Use the titrations on the web site (move the slider bar to. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Calculate. Buffer Example Problems.
From www.youtube.com
Challenging Buffer Problems worked out YouTube Buffer Example Problems Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared.. Buffer Example Problems.
From www.slideserve.com
PPT Processes PowerPoint Presentation, free download ID482301 Buffer Example Problems A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex {1}\)). 1) to solve the above. Systematic solution to buffer problems. Calculate the ph of the solution that results from the addition of. Buffer Example Problems.
From www.youtube.com
17.2 Calculating pH of Buffer Solutions YouTube Buffer Example Problems Calculate the ph of the solution that results from the addition of 0.040 moles of hno 3 to a buffer made by combining 0.500 l of 0.380. Use the titrations on the web site (move the slider bar to. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Systematic solution to buffer problems. Buffer solutions. Buffer Example Problems.
From www.studocu.com
Buffer PracticeKey Practice Worksheet key Buffer Practice Problems Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. 1) to solve the above. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Systematic solution to buffer problems. Calculate the ph of an unbuffered 0.010m acetic acid solution. Calculate the ph. Buffer Example Problems.
From studylib.net
Buffer Problems with answers Buffer Example Problems This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Systematic solution to buffer problems. Use the titrations on the web site (move the slider bar to. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. 1) to solve the above. Buffer. Buffer Example Problems.