Calorimetry Practice Problems Pdf . problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. Conceptual questions 1) draw and label a diagram of a. As always, include work and show the units to ensure full credit. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Use the information in this chart to answer the following questions: solve the following problems. What is the spepipc heat of *luminum if the temperature of a 28.4. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. A 445 g sample of ice at. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,.
from www.chegg.com
calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Use the information in this chart to answer the following questions: problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. As always, include work and show the units to ensure full credit. A 445 g sample of ice at. What is the spepipc heat of *luminum if the temperature of a 28.4. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. solve the following problems. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
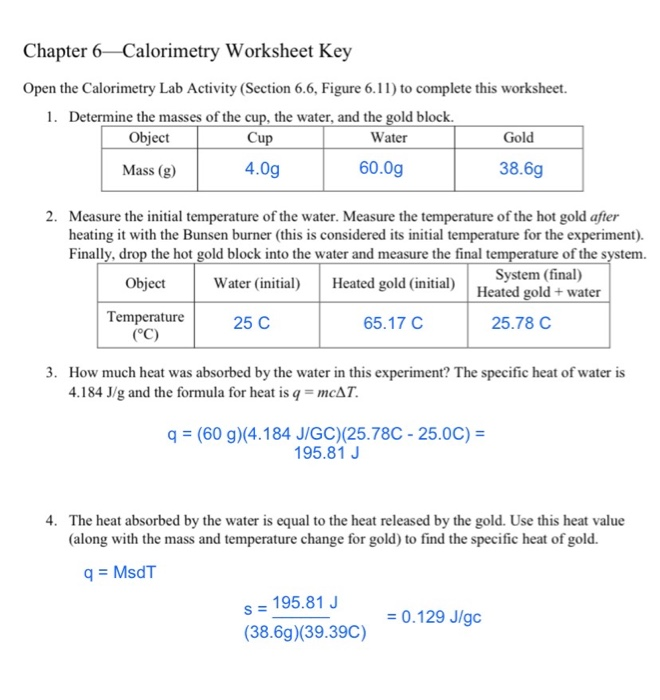
Solved Chapter 6 Calorimetry Worksheet Key Open the
Calorimetry Practice Problems Pdf calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. solve the following problems. Conceptual questions 1) draw and label a diagram of a. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? A 445 g sample of ice at. As always, include work and show the units to ensure full credit. What is the spepipc heat of *luminum if the temperature of a 28.4. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. Use the information in this chart to answer the following questions: in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,.
From www.youtube.com
Calorimetry Practice problems 1 YouTube Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? As always, include work and show the units to ensure full credit. What is the spepipc heat of *luminum if the temperature of a 28.4. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. Conceptual questions 1) draw and label. Calorimetry Practice Problems Pdf.
From exyqlzddm.blob.core.windows.net
Calorimeter Examples Chemistry at Sima Montgomery blog Calorimetry Practice Problems Pdf solve the following problems. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. A 445 g sample of ice at. As always, include work and show the units to ensure full credit. calorimetry practice problems specific heat for water =. Calorimetry Practice Problems Pdf.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. As always, include work and show the units to ensure full credit. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. How much energy is needed to change the temperature of 50.0 g of. Calorimetry Practice Problems Pdf.
From teachsimple.com
Calorimetry Practice by Teach Simple Calorimetry Practice Problems Pdf Conceptual questions 1) draw and label a diagram of a. What is the spepipc heat of *luminum if the temperature of a 28.4. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s),. Calorimetry Practice Problems Pdf.
From devonnewsrichardson.blogspot.com
Specific Heat and Calorimetry Worksheet Answer Key Calorimetry Practice Problems Pdf 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. solve the following problems. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. calorimetry practice problems specific heat for water = 4.184. Calorimetry Practice Problems Pdf.
From studylib.net
Calorimetry Problems Calorimetry Practice Problems Pdf A 445 g sample of ice at. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. What is the spepipc heat of *luminum if the temperature of a 28.4. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter. Calorimetry Practice Problems Pdf.
From www.scribd.com
Bomb Calorimetry Practice Problems PDF Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. What is the spepipc heat of *luminum if the. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Practice Problems PDF Calorie Chemistry Calorimetry Practice Problems Pdf A 445 g sample of ice at. What is the spepipc heat of *luminum if the temperature of a 28.4. Conceptual questions 1) draw and label a diagram of a. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? solve the following problems. problem \(\pageindex{8}\) when 1.0 g of fructose, c. Calorimetry Practice Problems Pdf.
From studylib.net
Specific Heat Capacity Practice Problems Calorimetry Practice Problems Pdf Conceptual questions 1) draw and label a diagram of a. As always, include work and show the units to ensure full credit. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Use the information in this chart to answer the following questions: 1) if 0.315 moles of hexane (c6h14) is combusted in a. Calorimetry Practice Problems Pdf.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimetry Practice Problems Pdf 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. As always, include work and show the units to ensure full credit. solve the following problems. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems specific heat for water =. Calorimetry Practice Problems Pdf.
From learningnovotyzy.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. solve the following problems. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Problems PDF Materials Quantity Calorimetry Practice Problems Pdf solve the following problems. What is the spepipc heat of *luminum if the temperature of a 28.4. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Conceptual questions 1) draw and label a diagram of a. in this set of practice questions, we will go over the main types of questions. Calorimetry Practice Problems Pdf.
From studylib.net
Calorimetry Problems Calorimetry Practice Problems Pdf solve the following problems. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. As always, include work and show the units to ensure full credit. in this set of practice questions, we will go over the. Calorimetry Practice Problems Pdf.
From studylib.net
CALORIMETER PRACTICE PROBLEMS 1 1. Find the specific Calorimetry Practice Problems Pdf problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems specific heat for water = 4.184 j/g0c. Calorimetry Practice Problems Pdf.
From lessoncampusstgeorge.z21.web.core.windows.net
Calorimetry Problems Worksheet With Answers Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. A 445 g sample of ice at. Use the information in this chart. Calorimetry Practice Problems Pdf.
From learningschoolconsoled.z14.web.core.windows.net
Coffee Cup Calorimeter Practice Problems Calorimetry Practice Problems Pdf As always, include work and show the units to ensure full credit. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems specific heat for water = 4.184. Calorimetry Practice Problems Pdf.
From www.youtube.com
Calorimetry Sample Problem YouTube Calorimetry Practice Problems Pdf calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. solve the following problems. in this set of practice questions, we will go over the main types of. Calorimetry Practice Problems Pdf.
From studylib.net
CALORIMETRY WORKSHEET 2 Calorimetry Practice Problems Pdf What is the spepipc heat of *luminum if the temperature of a 28.4. solve the following problems. Conceptual questions 1) draw and label a diagram of a. Use the information in this chart to answer the following questions: calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. problem \(\pageindex{8}\) when 1.0 g of fructose,. Calorimetry Practice Problems Pdf.
From studylib.net
Calorimetry practice Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? solve the following problems. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Practice Problems WS2 PDF Calorie Heat Calorimetry Practice Problems Pdf A 445 g sample of ice at. What is the spepipc heat of *luminum if the temperature of a 28.4. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,.. Calorimetry Practice Problems Pdf.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Practice Problems Pdf calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. A 445 g sample of ice at. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. As always, include work and show the units to ensure full credit. solve the following problems. . Calorimetry Practice Problems Pdf.
From www.scribd.com
3 Calorimetry Problems PDF Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. As always, include work and show the units to ensure full credit. How much energy is needed to change the. Calorimetry Practice Problems Pdf.
From goimages-egg.blogspot.com
Calorimetry Practice Problems Worksheet Answers Goimages Egg Calorimetry Practice Problems Pdf Conceptual questions 1) draw and label a diagram of a. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. As always, include work and show the units to ensure full credit. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? solve the following problems. Use the information in. Calorimetry Practice Problems Pdf.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. Use the information in this chart to answer the following questions: solve the following problems. What is the spepipc heat of *luminum if the temperature of a 28.4. How much energy is needed to change the temperature. Calorimetry Practice Problems Pdf.
From www.scribd.com
Specific Heat and Calorimetry Problems PDF Calorimetry Practice Problems Pdf 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. Use the information in this chart to answer the following questions: How much energy is. Calorimetry Practice Problems Pdf.
From materialschreiner.z19.web.core.windows.net
Solving A Basic Calorimetry Problem Aleks Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. solve the following problems. As always, include work and show the units. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Practice Worksheet PDF Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the spepipc heat of *luminum if the temperature of a 28.4. Conceptual questions 1) draw and label a diagram of a. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calorimetry practice. Calorimetry Practice Problems Pdf.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimetry Practice Problems Pdf How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the spepipc heat of *luminum if the temperature of a 28.4. solve the following problems. A 445 g sample of ice at. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found. Calorimetry Practice Problems Pdf.
From www.studocu.com
Calorimetry worked problems Calorimetry Problems A water Calorimetry Practice Problems Pdf solve the following problems. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the spepipc heat of *luminum if the temperature of a 28.4. A 445 g sample of ice at. As always, include work and show. Calorimetry Practice Problems Pdf.
From hxexppvfq.blob.core.windows.net
Ap Chemistry Calorimetry Problems at Christopher Redmond blog Calorimetry Practice Problems Pdf A 445 g sample of ice at. solve the following problems. Conceptual questions 1) draw and label a diagram of a. What is the spepipc heat of *luminum if the temperature of a 28.4. Use the information in this chart to answer the following questions: problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetryproblems PDF Heat Temperature Calorimetry Practice Problems Pdf problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. A 445 g sample of ice at. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go. Calorimetry Practice Problems Pdf.
From worksheetmediadwaum.z14.web.core.windows.net
Calorimetry Problems With Solutions Calorimetry Practice Problems Pdf Use the information in this chart to answer the following questions: How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? A 445 g sample of ice at. solve the following problems. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Practice Problems PDF Heat Properties Of Water Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. solve the following problems. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. How much energy is needed to. Calorimetry Practice Problems Pdf.
From www.scribd.com
Calorimetry Sample Problems PDF Calorimetry Practice Problems Pdf calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Conceptual questions 1) draw and label a diagram of a. As always, include work and show the units to ensure full credit. How much energy is needed to change. Calorimetry Practice Problems Pdf.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Calorimetry Practice Problems Pdf in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. problem \(\pageindex{8}\) when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a. What is the spepipc heat of *luminum if the temperature of. Calorimetry Practice Problems Pdf.