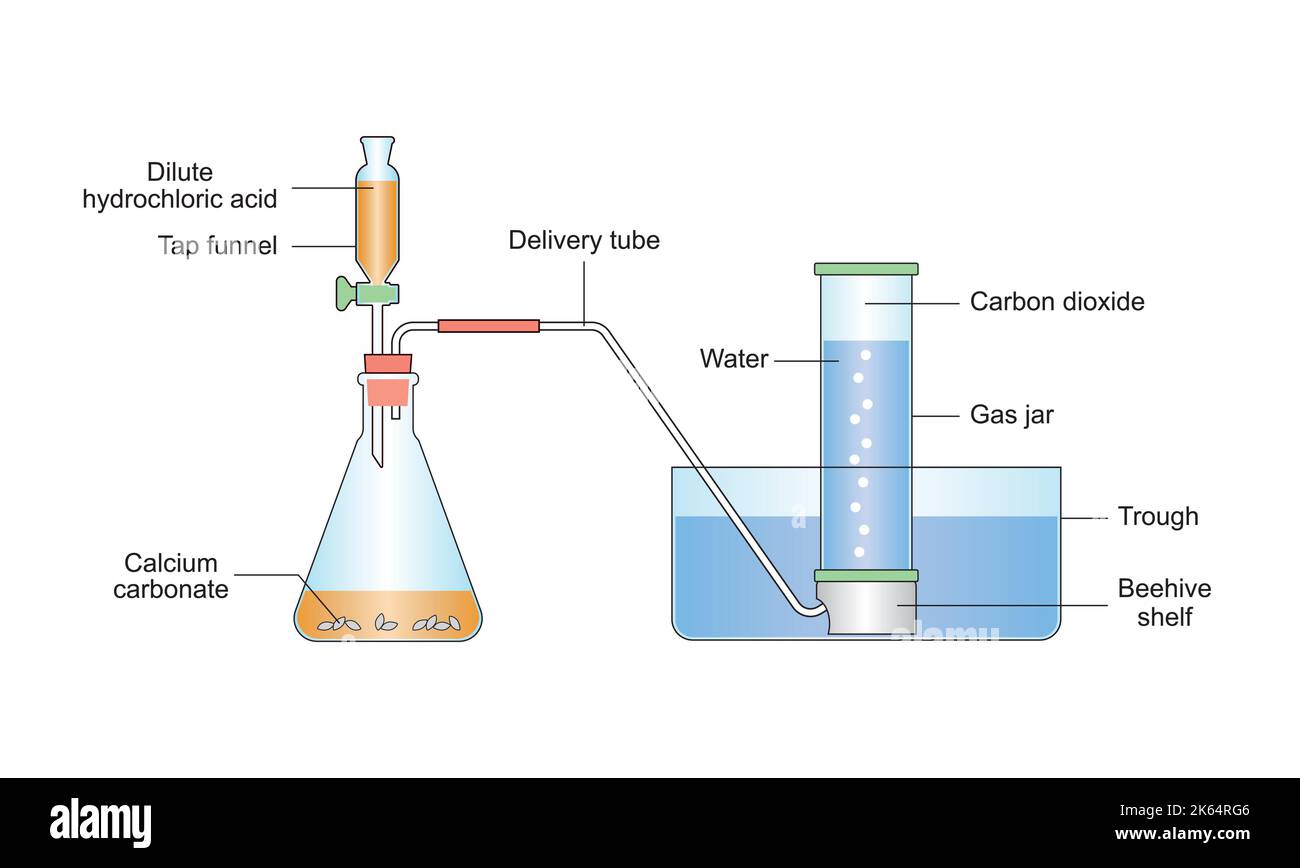
Hydrochloric Acid Laboratory Preparation . Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or.
from www.alamy.com
remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various.
Labelled diagram Stock Vector Images Alamy
Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride.
From www.vecteezy.com
Preparation of chlorine at room temparature in laboratory. vector image Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. In the laboratory, as well as on commercial. remember that the molarity of hydrochloric acid is equal to the. Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
Fully labelled diagram of the laboratory preparation of hydrogen Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will. Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
Hydrochloric acid bottle hires stock photography and images Alamy Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. . Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
A bottle of Hydrochloric acid (36 molarity 11.64, laboratory grade Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. In the laboratory, as well as on commercial. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid.. Hydrochloric Acid Laboratory Preparation.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Hydrochloric Acid Laboratory Preparation In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. remember that the molarity of hydrochloric acid is equal to the normality. Hydrochloric Acid Laboratory Preparation.
From buildupeducation.com
Laboratory method of preparation of hydrochloric acid Hydrochloric Acid Laboratory Preparation Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. In the laboratory, as. Hydrochloric Acid Laboratory Preparation.
From www.pinterest.com
Pin on Mansion Labs Hydrochloric Acid Laboratory Preparation remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Using a direct titration procedure, you will prepare and standardize a. Hydrochloric Acid Laboratory Preparation.
From askfilo.com
D. PREPARATION Of Hydrochloric acid Laboratory Method Hydrochloric ac.. Hydrochloric Acid Laboratory Preparation In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. . Hydrochloric Acid Laboratory Preparation.
From www.vecteezy.com
Preparation of Hydrochloric Acid in Laboratory . Hydrochloric acid is Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock. Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
Labelled diagram Stock Vector Images Alamy Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Hydrogen chloride is produced. Hydrochloric Acid Laboratory Preparation.
From www.youtube.com
Preparation and Standardization of 0.1 N Hydrochloric Acid YouTube Hydrochloric Acid Laboratory Preparation Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or.. Hydrochloric Acid Laboratory Preparation.
From www.vrogue.co
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric A vrogue.co Hydrochloric Acid Laboratory Preparation remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. Hydrogen chloride is produced in the laboratory and on a commercial scale. Hydrochloric Acid Laboratory Preparation.
From www.chemicals.co.uk
The Science Behind Hydrochloric Acid The Chemistry Blog Hydrochloric Acid Laboratory Preparation to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. In the laboratory, as well as on commercial. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. . Hydrochloric Acid Laboratory Preparation.
From fphoto.photoshelter.com
science chemistry hydrochloric acid Fundamental Photographs The Art Hydrochloric Acid Laboratory Preparation to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. In the laboratory, as well as on commercial. remember that the molarity of hydrochloric acid is equal to the normality of the solution,. Hydrochloric Acid Laboratory Preparation.
From discover.hubpages.com
How to Prepare Pure Hydrochloric Acid for the Home Lab HubPages Hydrochloric Acid Laboratory Preparation In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct. Hydrochloric Acid Laboratory Preparation.
From www.adda247.com
Hydrochloric Acid Formula, HCL Density, Chemical Name, Uses Hydrochloric Acid Laboratory Preparation remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide.. Hydrochloric Acid Laboratory Preparation.
From www.knowledgeboat.com
In the laboratory preparation of hydrochloric acid, hydrogen Hydrochloric Acid Laboratory Preparation to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. Using a direct titration. Hydrochloric Acid Laboratory Preparation.
From icsechemistry16.blogspot.com
laboratory preparation of HCl gas Hydrochloric Acid Laboratory Preparation remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Hydrogen chloride is. Hydrochloric Acid Laboratory Preparation.
From www.dreamstime.com
Preparation of Hydrogen Gas in Laboratory with the Help of Zinc and Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which. Hydrochloric Acid Laboratory Preparation.
From www.youtube.com
How to prepare 1N Hydrochloric acid (HCl)? YouTube Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. In the laboratory, as well as on commercial. Hydrogen chloride is produced in the laboratory and on a commercial scale. Hydrochloric Acid Laboratory Preparation.
From www.w3schools.blog
Preparation of Hydrogen W3schools Hydrochloric Acid Laboratory Preparation remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. to prepare one. Hydrochloric Acid Laboratory Preparation.
From www.walmart.com
Hydrochloric Acid, 6 M, Laboratory Grade, 500 Ml Hydrochloric Acid Laboratory Preparation to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in. Hydrochloric Acid Laboratory Preparation.
From www.indiamart.com
Liquid Hydrochloric Acid, 99, Bottle at Rs 200/bottle in Hyderabad Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. remember that the molarity of hydrochloric acid is. Hydrochloric Acid Laboratory Preparation.
From www.dreamstime.com
Preparation of Carbondioxide Gas in Laboratory with the Help of Calcium Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. In. Hydrochloric Acid Laboratory Preparation.
From www.shutterstock.com
Preparation Hydrochloric Acid Laboratory Vector Image Stock Vector Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. In the laboratory, as well as on commercial. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. to prepare one liter of 1n solution hydrochloric. Hydrochloric Acid Laboratory Preparation.
From www.youtube.com
6M HCl ( hydrochloric acid) preparation from 37 stock ! YouTube Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. In the laboratory, as well as on commercial. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium. Hydrochloric Acid Laboratory Preparation.
From www.studypool.com
SOLUTION Experiment preparation and standardization of 0.1 m HCL Hydrochloric Acid Laboratory Preparation In the laboratory, as well as on commercial. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Hydrogen chloride is produced in the laboratory and on a commercial scale. Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
Diagram of the laboratory preparation of carbon dioxide from Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock. Hydrochloric Acid Laboratory Preparation.
From www.ethosbiosciences.com
Hydrochloric Acid, Concentrated • Ethos Biosciences Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. remember that the molarity of hydrochloric acid. Hydrochloric Acid Laboratory Preparation.
From www.scribd.com
Standardization of Hydrochloric Acid Titration Hydrochloric Acid Hydrochloric Acid Laboratory Preparation prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. remember that the molarity of hydrochloric acid is equal to the normality of the solution, which means 1m solution is also a 1n solution. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and. Hydrochloric Acid Laboratory Preparation.
From www.vedantu.com
Explain the method of preparation of hydrogen gas in the laboratory Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. prepare a 0.01n hydrochloric acid (hcl) solution with this detailed guide. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. In the laboratory, as well as on commercial. Hydrogen chloride is produced in the laboratory and on. Hydrochloric Acid Laboratory Preparation.
From www.knowledgeboat.com
In the laboratory preparation of hydrochloric acid, hydrogen Hydrochloric Acid Laboratory Preparation In the laboratory, as well as on commercial. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml. Hydrochloric Acid Laboratory Preparation.
From www.alamy.com
preparation of hydrochloric acid by dissolving hydrogen chloride in Hydrochloric Acid Laboratory Preparation Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. hydrochloric acid (hcl) is. Hydrochloric Acid Laboratory Preparation.
From www.vecteezy.com
Preparation of chlorine in laboratory. vector image illustration Hydrochloric Acid Laboratory Preparation hydrochloric acid (hcl) is a strong, corrosive acid commonly used in clinical laboratories for various. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. remember that the molarity. Hydrochloric Acid Laboratory Preparation.
From brainly.in
OR(1) Draw a labelled diagram to show the preparation of Hydrochloric Acid Laboratory Preparation to prepare one liter of 1n solution hydrochloric acid, mix 82 ml of 37% (w/w) stock solution with 918 ml of distilled or. Hydrogen chloride is produced in the laboratory and on a commercial scale by heating sodium chloride. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. remember that the molarity. Hydrochloric Acid Laboratory Preparation.