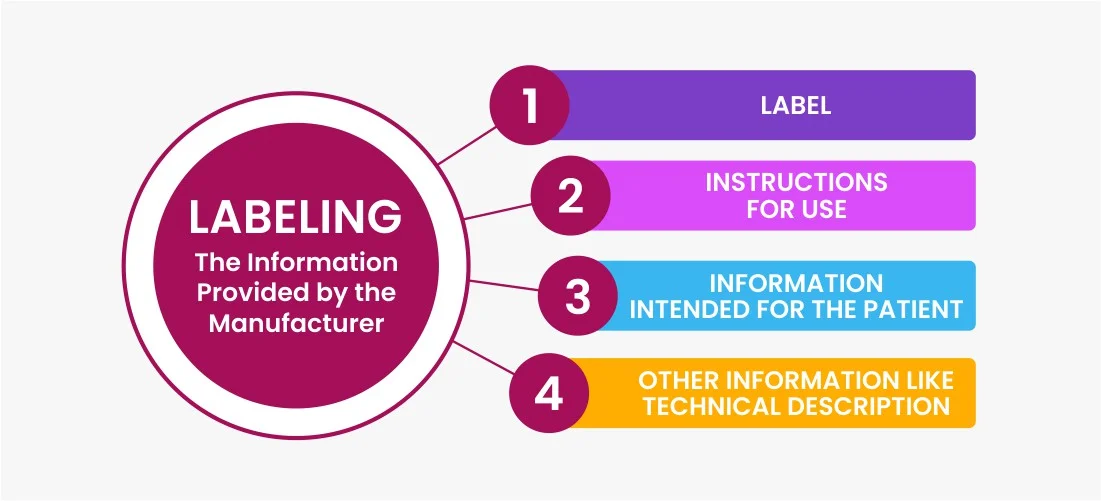
Medical Device Label Guidance . the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. these translations should be used as a guide only. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations.
from medicaldevicelicense.com
Principles of labelling for medical. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. these translations should be used as a guide only. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.
EU MDR Medical Device Labeling RequirementsA Complete Guide
Medical Device Label Guidance Principles of labelling for medical. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations should be used as a guide only. Principles of labelling for medical.
From www.pinterest.ca
Guidance Document Guidance for the Labelling of Medical Devices, not Medical Device Label Guidance labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should be used as a guide only. Principles of labelling for medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. this guidance document describes the general. Medical Device Label Guidance.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Medical Device Label Guidance these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’). Medical Device Label Guidance.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following. Medical Device Label Guidance.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations should be used as. Medical Device Label Guidance.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Medical Device Label Guidance these translations should be used as a guide only. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and. Medical Device Label Guidance.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of. Medical Device Label Guidance.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Label Guidance Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should be used as a guide only. this guidance assists manufacturers. Medical Device Label Guidance.
From www.schlafenderhase.com
A Guide to Medical Device Labeling Requirements Schlafender Hase Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should be used as a guide only. labeling regulations pertaining to medical devices are. Medical Device Label Guidance.
From hxelpllny.blob.core.windows.net
Medical Device Labeling Uk at Tomas Prather blog Medical Device Label Guidance the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. . Medical Device Label Guidance.
From giotbxaec.blob.core.windows.net
Software As A Medical Device Labeling Requirements at Steven Osborne blog Medical Device Label Guidance Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance applies to. Medical Device Label Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Guidance this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance document describes the general labelling principles for medical devices. Medical Device Label Guidance.
From www.vrogue.co
Fda Medical Device Labeling Regulations Archives Medi vrogue.co Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should be used as a guide only. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that. Medical Device Label Guidance.
From data1.skinnyms.com
Medical Device Label Template Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations. Medical Device Label Guidance.
From www.jointcommission.org
Symbol table for device labeling Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. . Medical Device Label Guidance.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. these translations should be used as a guide only. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance assists manufacturers in their development, and assist. Medical Device Label Guidance.
From exeedqm.com
How FDA is Shaping a Regulatory Policy for Device Cybersecurity — Exeed Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. these translations should be used as a guide only. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various. Medical Device Label Guidance.
From www.alamy.com
Full set of medical device packaging symbols with warning information Medical Device Label Guidance the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. Principles of labelling for medical. this. Medical Device Label Guidance.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Medical Device Label Guidance these translations should be used as a guide only. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Principles of labelling for medical. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance applies to. Medical Device Label Guidance.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Medical Device Label Guidance the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. Principles of labelling for medical. . Medical Device Label Guidance.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Label Guidance labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. . Medical Device Label Guidance.
From www.barcode-us.com
Medical Devices UDI Medical Device Label Guidance Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. these. Medical Device Label Guidance.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Medical Device Label Guidance this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should. Medical Device Label Guidance.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Label Guidance labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. this guidance. Medical Device Label Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Guidance the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should be used as a guide only. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing,. Medical Device Label Guidance.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Label Guidance this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of title. Medical Device Label Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of. Medical Device Label Guidance.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. these translations should be used as a guide only. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Medical Device Label Guidance.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Label Guidance these translations should be used as a guide only. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this guidance applies to the regulatory pathways of clinical. Medical Device Label Guidance.
From knobbemedical.com
FDA Issues Final Rule on Use of Symbols in Labeling Knobbe Medical Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should be used as a guide only. this guidance assists manufacturers in their development, and assist center. Medical Device Label Guidance.
From www.morningtrans.com
International Medical Device Labeling Guide Morningside Medical Device Label Guidance the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. these translations should be used as a guide only. this guidance applies to the regulatory pathways of clinical. Medical Device Label Guidance.
From www.regdesk.co
HSA Guidance on UDI System Components and Labeling RegDesk Medical Device Label Guidance this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Principles of labelling for medical. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should. Medical Device Label Guidance.
From barcode-labels.com
Medical Devices Electronic Imaging Materials Medical Device Label Guidance Principles of labelling for medical. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance applies to the regulatory pathways of clinical trial approval, manufacturing, and import licensing. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information. Medical Device Label Guidance.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Guidance Principles of labelling for medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should be used as a guide only. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance assists manufacturers. Medical Device Label Guidance.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Label Guidance labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. these translations should be used as a guide only. this guidance assists manufacturers in their development, and assist. Medical Device Label Guidance.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Label Guidance Principles of labelling for medical. these translations should be used as a guide only. this guidance assists manufacturers in their development, and assist center reviewers in their review and evaluation of medical. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labeling regulations pertaining to medical devices are. Medical Device Label Guidance.